
��֪���ٳ��³�ѹ�£�������O3��Ϊ��ɫ���壻��O3�к�ǿ������ ��Ũ�ȳ���ʱ�������ж������ã����ڵ��������O2��ת��ΪO3��N2Ҳ����O2��Ӧ����KI�ᱻǿ����������Ϊ���ʵ⣬���ʵ������۱�������Ʒ����ҺΪ��ɫ����ǿ���������Ϊ��ɫ��
��Ũ�ȳ���ʱ�������ж������ã����ڵ��������O2��ת��ΪO3��N2Ҳ����O2��Ӧ����KI�ᱻǿ����������Ϊ���ʵ⣬���ʵ������۱�������Ʒ����ҺΪ��ɫ����ǿ���������Ϊ��ɫ��
ʵ�����ṩ����װ�ú�ҩƷ�������O3���Ʊ���������֤ʵ�顣

��1������װ�õ�����˳��Ϊ_______��_______��_______��______
��2��B�з�����Ӧ�Ļ�ѧ����ʽΪ________________________________
��3��ʵ�鿪ʼʱ��Ҫ��ͨһ��ʱ���������ٺ��ϵ�Դ���ؿ�ʼ����O3��ԭ���ǣ�
______________________________________________________________________
��4�����Ͽ���һ��ʱ���Dװ���г��ֵ�������_______________________________
��5���Ӱ�ȫ�������Ƕȿ���Ӧ���ӵ�װ����__________________________װ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E��X��Y��Z���dz��л�ѧ�еij������ʣ�����A����Ȼ������Ҫ�ɷ֣�X��Y��Z�ǵ��ʣ�B��C��D��E�������������C��Һ�壬Y�Ǻ�ɫ���壬E�Ǻ���ɫ��ĩ������֮�������·�Ӧ��ϵ��

�����������Ϣ�ش��������⣺
��1���й����ʵĻ�ѧʽ��AΪ____��____��CΪ___��___��
��2��B��Y��Ӧ�Ļ�ѧ����ʽΪ_____________��______________��
D��E��Ӧ�Ļ�ѧ����ʽΪ_____________��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�û�ѧ�����ʾ��
(1)ˮ����Ԫ�صĻ��ϼ� ��
(2) 3�������� ��
(3)�������Һ�е�������_____ ___��
(4) 2�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڻ�ѧ��ӦaX+bY=cM+dN��X��Y��M��N��ʾ��Ӧ���������Ļ�ѧʽ��a��b��c��dΪ��ƽϵ����������˵������ȷ����
A��a+b��һ������c+d B��X��Y�ķ�����֮��һ������M��N������֮��
C��X��Y������ԭ�Ӹ���֮��һ������M��N��ԭ�Ӹ���֮��
D��X��Y����Ԫ������һ����M��N������Ԫ��������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�еġ����ڿθġ��У����л�ѧ���롰���ʵ������Ľ�ѧ���ݣ����ʵ�����һ���µ����������䵥λ��Ħ����mol����1Ħ�����ʴ�Լ����6.02��1023�������ʵ�������1mol��������6.02��1023��H2���ӣ�����2��6.02��1023��Hԭ�ӣ��ݴ˻ش�
��1�����ʵ��������ʵ�������__________�����ͬ������ͬ������������
��2��1mol���ᣨH2SO4���к���_________����ԭ�ӣ�����_______����ԭ�ӡ�
��3��1mol������O3�����____ ___������
___������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У���������ȼ�գ���Ӧ��������ķ�����֮��Ϊ1��3���ǣ� ��
A.CH4 B.C2H2 C.C2H6O D.C2H6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
|
 ������������Ʒ�����ݲ��������ȷ���л������ɣ���֪��C�е�����ͭ��ȷ���л����е�̼Ԫ����ȫת��Ϊ������̼��A�еķ�ӦΪ��2H2O=====2H2O+O2����
������������Ʒ�����ݲ��������ȷ���л������ɣ���֪��C�е�����ͭ��ȷ���л����е�̼Ԫ����ȫת��Ϊ������̼��A�еķ�ӦΪ��2H2O=====2H2O+O2���� 
�ش��������⣺
��1��Aװ����a����������________��b��ʢ�ŵ�������___________��
��2����ȥ��Bװ�û��ʲôԪ�صIJ��������Ӱ�죿______����ʹ�������_______���ƫ��ƫС������
��3��Eװ������ʢ���������ѡ��__________����ѡ�������������Һ��������������Һ����ˮ��
��4����ȷ��ȡ1.12g��Ʒ����Ʒֻ��C��H��O����Ԫ���е����ֻ����֣��������ȼ�գ�Dװ������1.44g��Eװ������3.52g������л�����Ʒ��������Ԫ��Ϊ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ��ʵ���������ȷ���ǣ� ��
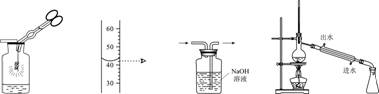
A.��˿��������ȼ�� B.��ȡҺ������ C.��ȥCO�е�CO2 D.��ȡ����ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
÷���в��ֵ����ز���������Ҫ�������漰��ѧ�仯����
A��ƽԶ���ե֭ B�������������� C��÷�ؽ������ D���廪ˮ��ɹ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com