
| �ձ��� | �ձ��� | �ձ��� | �ձ��� | �ձ��� | |
| ������Ʒ������/g | 10 | 10 | 10 | 10 | 10 |
| ����ϡ���������/g | 10 | 20 | 30 | 40 | 50 |
| ��ַ�Ӧ���������������/g | 0.88 | 1.76 | 2.64 | 3.52 | 3.52 |
���� ��1�������ձ����������ݣ��ɵ�֪ÿ����10gϡ������ȫ��Ӧ�ɷų�����0.88g��������ϡ����50gʱ�ų�������̼���������ȴΪ3.52g����0.88g��5�������жϴ�ʱ̼�������ȫ��Ӧ��
��2������̼���������ϡ������ȫ��Ӧ�ų�������̼������3.52g���ɷ�Ӧ�Ļ�ѧ����ʽ����̼��Ƶ�������Ȼ�����������������㹫ʽ�������Ʒ��̼��Ƶ�����������
��� �⣺��1���ɱ������ݿɵ�֪��ÿ����10gϡ������ȫ��Ӧ�ɷų�����0.88g�����ֱ����40g��50gϡ����ʱ�ų�������̼��������Ϊ3.52g����10g��Ʒ������ϡ���ᷴӦ������ɶ�����̼����3.52g��
�ʴ�Ϊ��3.52��
��2����10g��Ʒ��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 3.52g
$\frac{100}{x}=\frac{44}{3.52g}$
x=8g
��Ʒ��̼��Ƶ���������=$\frac{8g}{10g}$��100%=80%
��
��1��3.52��
��2����Ʒ��̼��Ƶ���������Ϊ80%��
���� ����ʵ������¼������ʱ��ע�����ݱ仯����Ĺ��ɣ����ñ仯�����ж���ȫ��Ӧʱ���漰���ʵ�������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
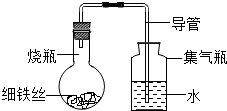
| ʱ��/Сʱ | 0 | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 |
| Aƿ��ʢ��ϸ��˿�� | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bƿ��ʢմ��ʳ��ˮ��ϸ��˿�� | 0 | 0.4 | 1.2 | 3.4 | 5.6 | 7.6 | 9.8 |
| Cƿ��ʢմ����ˮ��ϸ��˿�� | 0 | 0 | 0 | 0.3 | 0.8 | 2.0 | 3.5 |
| Dƿ��ϸ��˿��ȫ��û��ʳ��ˮ�У� | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������պ�����Һ�е��� | B�� | ��п��ϡ���ᷴӦ��ȡ���� | ||
| C�� | �Ŵ�������Ʒ�ܱ������� | D�� | ͭ�㷺�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH4NO3���� | B�� | ��ʯ�� | C�� | ��ʯ�� | D�� | ʳ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com