



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢ݢ� | B���ۢ� |
| C���ۢܢ� | D���ݢߢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| A����ʵ����������ϣ��˽�ʳ��������ʱ���ܽ�� |
| B���������У���δ�ܳ���ܽ⣬��ʵ��IJ��ʽ�ƫ�� |
| C���������У��õ��������Ǵ����е����������� |
| D������V�У��������Һ������Ϊֹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ�Dz�������������������ʵ��װ�ã��ر�ֹˮ�У�ͨ��ʹ����ȼ�գ���ش��������⣺
��ͼ�Dz�������������������ʵ��װ�ã��ر�ֹˮ�У�ͨ��ʹ����ȼ�գ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
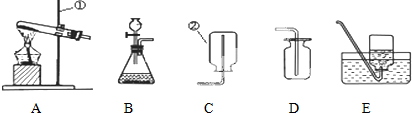
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��һ������CaCl2��HCl�����Һ����μ�����������Ϊ10%��Na2CO3��Һ����Ӧ�����м����Na2CO3��Һ����������������������������ϵ��ͼ��ʾ��
��һ������CaCl2��HCl�����Һ����μ�����������Ϊ10%��Na2CO3��Һ����Ӧ�����м����Na2CO3��Һ����������������������������ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ����ѧ��ѧ��ȤС��Ϊ�ⶨijʯ��ʯ��Ʒ�����ʲ�����ˮ��Ҳ�����ᷴӦ����̼��Ƶ�����������������ͼ��ʾ��ʵ�飮
ʵ����ѧ��ѧ��ȤС��Ϊ�ⶨijʯ��ʯ��Ʒ�����ʲ�����ˮ��Ҳ�����ᷴӦ����̼��Ƶ�����������������ͼ��ʾ��ʵ�飮 �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | X | Y | Z | Q |
| ��Ӧǰ����/g | 8 | 2 | 40 | 5 |
| ��Ӧ������/g | ���� | 24 | 8 | 23 |
| A���٢ڢ� | B���ۢ� |
| C���ۢܢ� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ˮ�DZ������Ȼ��Դ��
ˮ�DZ������Ȼ��Դ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com