
| ��Ӧǰ | ��Ӧ�� | ||
| ʵ������ | ����п����Ʒ������ | ����ϡ����������������� | ʣ����������� |
| 10g | 50g | 59.8g | |
| 65 |
| x |
| 2 |
| 0.2g |
| 6.5g |
| 10g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� ��ͼ���ʹ�����Ϩ��˵��CO2�Ȳ�ȼ��Ҳ��֧��ȼ�գ����ܶȱȿ����Ĵ� |
B�� ��ͼ���¶ȸߵͶԷ��ӵ������˶�û��Ӱ�� |
C�� ��ͼ��ˮ�ϵİ���ȼ�գ�˵����߱���ȼ�յ��������� |
D��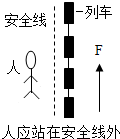 ��ͼ����Ϊ����ѹǿ�Ĵ�С�������йأ�������Ӧվ�ڰ�ȫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
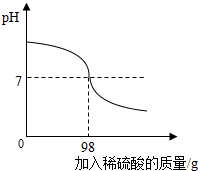 ��ֽ��������������������Ƶķ�ˮ���辭���������Ժ��ŷţ�Ϊ�ⶨ�˷�ˮ���������Ƶ�����������С��ȡ80g��ˮ��Ʒ���뵽��ƿ�У���μ���10%��ϡ���ᣬ��ͼ��ʾ��
��ֽ��������������������Ƶķ�ˮ���辭���������Ժ��ŷţ�Ϊ�ⶨ�˷�ˮ���������Ƶ�����������С��ȡ80g��ˮ��Ʒ���뵽��ƿ�У���μ���10%��ϡ���ᣬ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
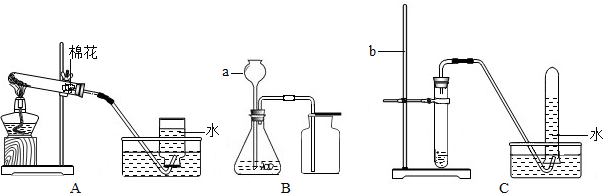
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
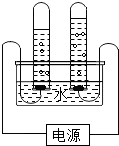 ��ͼ�ǵ��ˮ�ļ���װ��ͼ��
��ͼ�ǵ��ˮ�ļ���װ��ͼ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com