
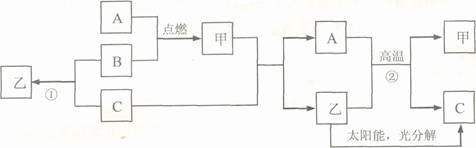
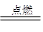 2H2O ²śĪļĪŽĪŪČ¾
2H2O ²śĪļĪŽĪŪČ¾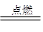 2H2O£®
2H2O£®

 ĆūŠ£ĶØŠŠÖ¤ÓŠŠ§×÷ŅµĻµĮŠ“š°ø
ĆūŠ£ĶØŠŠÖ¤ÓŠŠ§×÷ŅµĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| Ń”Ļī | ĪļÖŹ | ŌÓÖŹ | ³żČ„ŌÓÖŹµÄ·½·Ø |
| A | CO2 | CO | µćČ¼ |
| B | KClO3 | KCl | ¼ÓČČ |
| C | NaOHČÜŅŗ | Na2CO3 | ¼ÓŹŹĮæCa(OH)2ČÜŅŗ£¬¹żĀĖ |
| D | FeCl3 | CuCl2 | ¼Ó¹żĮæĢś·Ū£¬¹żĀĖ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ĻņijĪŽÉ«ČÜŅŗÖŠµĪ¼Ó·ÓĢŖŹŌŅŗĪŽĻÖĻó£¬ŌņČÜŅŗŅ»¶Ø³ŹÖŠŠŌ |
| B£®Ļņij¹ĢĢåÖŠµĪ¼ÓĻ”ŃĪĖį£¬ÓŠĘųÅŻ²śÉś£¬øĆ¹ĢĢåŅ»¶ØŹĒĢ¼ĖįŃĪ |
| C£®12mLÕōĮóĖ®ŗĶ20mL¾Ę¾«»ģŗĻŗó£¬Ģå»żŠ”ÓŚ32mL£¬ĖµĆ÷·Ö×ÓÖ®¼äÓŠ¼äĻ¶ |
| D£®½«Ļ“µÓ¼ĮµĪ¼Óµ½ÉŁĮæÖ²ĪļÓĶŗĶĖ®µÄ»ģŗĻĪļÖŠ£¬Õńµ“”¢¾²ÖĆ£¬²»·Ö²ć£¬ĖµĆ÷Ö²ĪļÓĶæÉČÜÓŚĖ® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
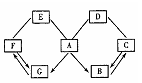
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĶʶĻĢā
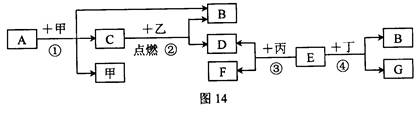
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŃéÖ¤NaOHČÜŅŗÖŠŹĒ·ńŗ¬ÓŠSO42-,ĻČ¼ÓČėĻ”ĮņĖį,ŌŁ¼ÓČėBaCl2ČÜŅŗ,ÓŠ°×É«³Įµķ,Ö¤Ć÷ŗ¬ÓŠSO42- |
| B£®²ā¶ØČÜŅŗµÄpH,ĻČÓĆĖ®ČóŹŖpHŹŌÖ½£¬Č»ŗó½«ŹŌÖ½²åČė“ż²āŅŗÖŠ |
| C£®ĻņijĪŽÉ«ČÜŅŗÖŠµĪČė·ÓĢŖŹŌŅŗ³ŹŗģÉ«£¬ĖµĆ÷øĆČÜŅŗŅ»¶ØŹĒ¼īČÜŅŗ |
| D£®µćČ¼æÉČ¼ŠŌĘųĢåŹ±,ŅŖĻČ¼ģŃéĘųĢå“æ¶Č,ŗóµćČ¼ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

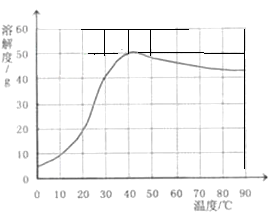
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
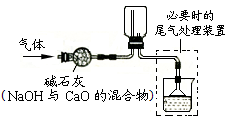
| A£®¢Ł¢Ś¢Ū | B£®¢Ś¢Ū¢Ż |
| C£®¢Ū¢Ü¢Ž | D£®¢Ł¢Ż¢Ž |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĶʶĻĢā
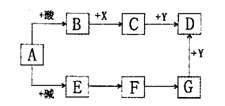
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com