
��4.5�֣���ʯ����һ�ְ�ɫ�Ŀ�״�Ӧ��ʮ�ֹ㷺��(1)��ʯ�ҵ���Ҫ�ɷ�Ϊ(�ѧʽ) ����ʳƷ��װ�У�������ʯ����������������ԭ����(�û�ѧ����ʽ��ʾ) ��
(2)��ũ�壬��ʯ��Ҳ������������������������ݳ�������ʯ����ˮ����20����ʯ���飬Ϳˢǽ��͵��档ijũ������ʯ��ֱ��������Ȧ��������һ��ʱ����������㲿���↑�ѣ��е������������ˡ�����ԭ���� ��
(3)�������������Ľ�״��Ҫ�������ã����ù��ã���û�����������ˣ���ԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
��4��ʢ�������õ��������γ�һ�����Գ�ȥ�����գ�������ϡ�����ȥ����ԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
��1����CaO CaO+H2O=Ca(OH)2
(2)����ʯ���е�CaO��ˮ��Ӧ���������ɵ��������ƾ��и�ʴ��
��3��Ca(OH)2+CO2=CaCO3+H2O (4)CaCO3+2HCl=CaCl2+H2O+CO2��
�������������(1)��ʯ�ҵ���Ҫ�ɷ�ΪCaO������ʯ����������������ԭ����CaO+H2O=Ca(OH)2��
(2)����ʯ��ֱ��������Ȧ��������һ��ʱ����������㲿���↑�ѣ��е������������ˡ�����ԭ������ʯ���е�CaO��ˮ��Ӧ���������ɵ��������ƾ��и�ʴ�ԣ�
(3)ʯ�������ʱ����ˣ������ı仯��Ca(OH)2+CO2=CaCO3+H2O��
��4��ʢ�������õ��������γ�һ�����Գ�ȥ�����գ�������ϡ�����ȥ����ԭ����CaCO3+2HCl=CaCl2+H2O+CO2����
���㣺��ʯ�ҵ����ʺ���;����ʯ�ҵ����ʺ���;����ѧ����ʽ����д��
��������ʯ�ҵ���Ҫ�ɷ�ΪCaO���������������ʯ�ҵijɷ����������ƣ���������еĶ�����̼��Ӧ������̼��ơ�


 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ѡ�� | A | B | C | D |
| ���� | ���� | �ݲ� | ���� | ������ |
| pH | 2.9��3.5 | 3.0��4.0 | 3.5��4.5 | 6.8-8.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
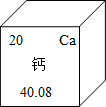 ��2013?����ģ�⣩���������У���Ԫ�صĴ��ڷdz��㷺��
��2013?����ģ�⣩���������У���Ԫ�صĴ��ڷdz��㷺���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��5�֣�����������ܽ�ȱ����ܽ�����ش��������⣺
��1��B��______���ܽ�����ߡ�
��2��40��ʱ���Ȼ��Ƶ��ܽ��______�����ڡ�С�ڻ���ڣ�����ص��ܽ�ȡ�
��3��������л����������Ȼ��ƣ���Ҫ�õ�����������صķ����� ��
��4����ʹ����صIJ�������Һת��Ϊ������Һ�����Բ�ȡ�ķ���֮һ�� ��
��5����ͼ��ʾ���ձ�A���DZ��͵�����������Һ�����ձ�B�м�����ʯ�Һ��ձ�A�б���ǣ����ܵ�ԭ����______������ţ���
A����Ӧ����ˮ��������������
B����Ӧ���ȣ��¶����ߣ����������ܽ�Ƚ���
C����ʯ����ˮ��Ӧ���ɵ��������Ʋ����ܽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ���꼶��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
��4.5�֣���ʯ����һ�ְ�ɫ�Ŀ�״�Ӧ��ʮ�ֹ㷺��(1)��ʯ�ҵ���Ҫ�ɷ�Ϊ(�ѧʽ) ����ʳƷ��װ�У�������ʯ����������������ԭ����(�û�ѧ����ʽ��ʾ) ��
(2)��ũ�壬��ʯ��Ҳ������������������������ݳ�������ʯ����ˮ����20����ʯ���飬Ϳˢǽ��͵��档ijũ������ʯ��ֱ��������Ȧ��������һ��ʱ����������㲿���↑�ѣ��е������������ˡ�����ԭ���� ��
(3)�������������Ľ�״��Ҫ�������ã����ù��ã���û�����������ˣ���ԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
��4��ʢ�������õ��������γ�һ�����Գ�ȥ�����գ�������ϡ�����ȥ����ԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com