
| �������� | ���� |
| ��H2O2��Һ������ʱ�����MnO2 | ����1��________��Ӱ�컯ѧ��Ӧ�����ʣ� |
| ʹ�õ���䴢��ʳ���ѱ��� | ����2���¶ȵĸߵ���Ӱ�컯ѧ��Ӧ�����ʣ� |
| ������ʱ��Ũ�����ϡ����Ҫ�� | ����3����Һ������������С��Ӱ�컯ѧ��Ӧ�����ʣ� |
| ��ú���ɷ���״����ȼ�� | ����4�����������Ĵ�С��Ӱ�컯ѧ��Ӧ�����ʣ� |
| ʵ�� | �¶� | ҩƷ | ���ݲ������� | ʵ����� | |
| �� | ������ | 5% H2O2 | ���������� | MnO2�ӿ��˷�Ӧ���ʣ� | |
| ������ | 5% H2O2 | MnO2 | �Ͽ� | ||
| �� | ������ | 5% H2O2 | MnO2 | �Ͽ� | �¶�Խ�ߣ���ѧ��Ӧ����Խ_____�� |
| ��ˮ�� | 5% H2O2 | MnO2 | �dz��� | ||
| �� | ������ | 5% H2O2 | MnO2 | �Ͽ� | ��Һ����������Խ��ѧ��Ӧ����Խ_____�� |
| ������ | 10% H2O2 | MnO2 | �dz��� | ||
| �� | ������ | ����ʯ����ĩ�� | 5% ���� | �Ͽ� | ��������Խ��ѧ��Ӧ����Խ______�� |
| ������ | ����ʯ�������� | 5% ���� | ���� | ||
| �������� | ���� |
| ��H2O2��Һ������ʱ�����MnO2 | ����1��������Ӱ�컯ѧ��Ӧ�����ʣ� |
| ʹ�õ���䴢��ʳ���ѱ��� | ����2���¶ȵĸߵ���Ӱ�컯ѧ��Ӧ�����ʣ� |
| ������ʱ��Ũ�����ϡ����Ҫ�� | ����3����Һ������������С��Ӱ�컯ѧ��Ӧ�����ʣ� |
| ��ú���ɷ���״����ȼ�� | ����4�����������Ĵ�С��Ӱ�컯ѧ��Ӧ�����ʣ� |
| ʵ�� | �¶� | ҩƷ | ���ݲ������� | ʵ����� | |
| �� | ������ | 5% H2O2 | ���������� | MnO2�ӿ��˷�Ӧ���ʣ� | |
| ������ | 5% H2O2 | MnO2 | �Ͽ� | ||
| �� | ������ | 5% H2O2 | MnO2 | �Ͽ� | �¶�Խ�ߣ���ѧ��Ӧ����Խ�죮 |
| ��ˮ�� | 5% H2O2 | MnO2 | �dz��� | ||
| �� | ������ | 5% H2O2 | MnO2 | �Ͽ� | ��Һ����������Խ��ѧ��Ӧ����Խ�죮 |
| ������ | 10% H2O2 | MnO2 | �dz��� | ||
| �� | ������ | ����ʯ����ĩ�� | 5% ���� | �Ͽ� | ��������Խ��ѧ��Ӧ����Խ�죮 |
| ������ | ����ʯ�������� | 5% ���� | ���� | ||
| ||


 Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �������� | ���� |
| ��H2O2��Һ������ʱ�����MnO2 | ����1��________��Ӱ�컯ѧ��Ӧ�����ʣ� |
| ʹ�õ���䴢��ʳ���ѱ��� | ����2���¶ȵĸߵ���Ӱ�컯ѧ��Ӧ�����ʣ� |
| ������ʱ��Ũ�����ϡ����Ҫ�� | ����3����Һ������������С��Ӱ�컯ѧ��Ӧ�����ʣ� |
| ��ú���ɷ���״����ȼ�� | ����4�����������Ĵ�С��Ӱ�컯ѧ��Ӧ�����ʣ� |
| ʵ�� | �¶� | ҩƷ | ���ݲ������� | ʵ����� | |
| �� | ������ | 5% H2O2 | ���������� | MnO2�ӿ��˷�Ӧ���ʣ� | |
| ������ | 5% H2O2 | MnO2 | �Ͽ� | ||
| �� | ������ | 5% H2O2 | MnO2 | �Ͽ� | �¶�Խ�ߣ���ѧ��Ӧ����Խ_____�� |
| ��ˮ�� | 5% H2O2 | MnO2 | �dz��� | ||
| �� | ������ | 5% H2O2 | MnO2 | �Ͽ� | ��Һ����������Խ��ѧ��Ӧ����Խ_____�� |
| ������ | 10% H2O2 | MnO2 | �dz��� | ||
| �� | ������ | ����ʯ����ĩ�� | 5% ���� | �Ͽ� | ��������Խ��ѧ��Ӧ����Խ______�� |
| ������ | ����ʯ�������� | 5% ���� | ���� | ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �������� | ���� |
| ��H2O2��Һ������ʱ�����MnO2 | ����1��________��Ӱ�컯ѧ��Ӧ�����ʣ� |
| ʹ�õ���䴢��ʳ���ѱ��� | ����2���¶ȵĸߵ���Ӱ�컯ѧ��Ӧ�����ʣ� |
| ������ʱ��Ũ�����ϡ����Ҫ�� | ����3����Һ������������С��Ӱ�컯ѧ��Ӧ�����ʣ� |
| ��ú���ɷ���״����ȼ�� | ����4�����������Ĵ�С��Ӱ�컯ѧ��Ӧ�����ʣ� |
| ʵ�� | �¶� | ҩƷ | ���ݲ������� | ʵ����� | |
| �� | ������ | 5% H2O2 | ���������� | MnO2�ӿ��˷�Ӧ���ʣ� | |
| ������ | 5% H2O2 | MnO2 | �Ͽ� | ||
| �� | ������ | 5% H2O2 | MnO2 | �Ͽ� | �¶�Խ�ߣ���ѧ��Ӧ����Խ_____�� |
| ��ˮ�� | 5% H2O2 | MnO2 | �dz��� | ||
| �� | ������ | 5% H2O2 | MnO2 | �Ͽ� | ��Һ����������Խ��ѧ��Ӧ����Խ_____�� |
| ������ | 10% H2O2 | MnO2 | �dz��� | ||
| �� | ������ | ����ʯ����ĩ�� | 5% ���� | �Ͽ� | ��������Խ��ѧ��Ӧ����Խ______�� |
| ������ | ����ʯ�������� | 5% ���� | ���� | ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫ʡģ���� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ɽ��ʡΫ��������з�����ѧ�п���ѧģ���Ծ��������������棩 ���ͣ������
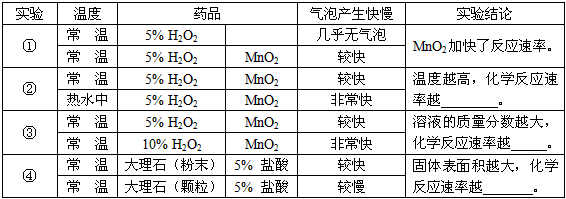
| �������� | ���� |
| ��H2O2��Һ������ʱ�����MnO2 | ����1��________��Ӱ�컯ѧ��Ӧ�����ʣ� |
| ʹ�õ���䴢��ʳ���ѱ��� | ����2���¶ȵĸߵ���Ӱ�컯ѧ��Ӧ�����ʣ� |
| ������ʱ��Ũ�����ϡ����Ҫ�� | ����3����Һ������������С��Ӱ�컯ѧ��Ӧ�����ʣ� |
| ��ú���ɷ���״����ȼ�� | ����4�����������Ĵ�С��Ӱ�컯ѧ��Ӧ�����ʣ� |
| ʵ�� | �¶� | ҩƷ | ���ݲ������� | ʵ����� | |
| �� | �� �� | 5% H2O2 | ���������� | MnO2�ӿ��˷�Ӧ���ʣ� | |
| �� �� | 5% H2O2 | MnO2 | �Ͽ� | ||
| �� | �� �� | 5% H2O2 | MnO2 | �Ͽ� | �¶�Խ�ߣ���ѧ��Ӧ����Խ_____�� |
| ��ˮ�� | 5% H2O2 | MnO2 | �dz��� | ||
| �� | �� �� | 5% H2O2 | MnO2 | �Ͽ� | ��Һ����������Խ��ѧ��Ӧ����Խ_____�� |
| �� �� | 10% H2O2 | MnO2 | �dz��� | ||
| �� | �� �� | ����ʯ����ĩ�� | 5% ���� | �Ͽ� | ��������Խ��ѧ��Ӧ����Խ______�� |
| �� �� | ����ʯ�������� | 5% ���� | ���� | ||
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com