
 =
=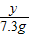 Y�T10.6g
Y�T10.6g Z�T11.7g
Z�T11.7g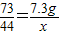 X�T4.4g
X�T4.4g ×100%�T24.2%
×100%�T24.2%

 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
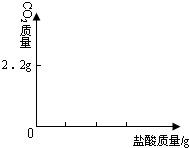
| ��� | ���� | NaOH��Һ | ��t/�� |
| 1 | 3.65% | 2.00% | 3.5 |
| 2 | 3.65% | 4.00% | x |
| 3 | 7.30% | 8.00% | 14 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
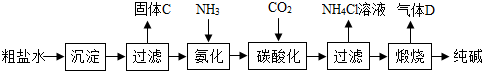
 �ᡢ������й㷺��;����Ҫ�����ij��ѧ�С���ͬѧΧ���⼸����������һϵ�е�̽�����
�ᡢ������й㷺��;����Ҫ�����ij��ѧ�С���ͬѧΧ���⼸����������һϵ�е�̽�����| ������ ������ |
OH- | NO3- | Cl- | SO42- | CO32- |
| H+ | �ܡ��� | �ܡ��� | �� | �ܡ��� | |
| Na+ | �� | �� | �� | �� | �� |
| Ba2+ | �� | �� | �� | ���� | ���� |
| ʵ��Ŀ�� | ʵ����� | ���� | ���ۻ�ѧ����ʽ |
| ��ȥ̼���� | ȡ�����ù�����Ʒ����ˮ�����Һ���μ������� BaCl2 BaCl2 ��Һ����ַ�Ӧ����� |
�а�ɫ�������� | �йط�Ӧ�Ļ�ѧ����ʽΪ Na2CO3+BaCl2�TBaCO3��+2NaCl Na2CO3+BaCl2�TBaCO3��+2NaCl |
| �����Ƿ����������� | ����Һ�еμӷ�̪��Һ | ��Һ��Ϊ��ɫ ��Һ��Ϊ��ɫ |
����Ʒ�к����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||

| 106 |
| 10.6g |
| 44 |
| x |
| 106 |
| 10.6g |
| 44 |
| x |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
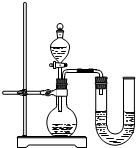
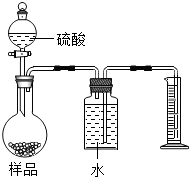 ��2011?�����ʼ죩���������ƹ����п��ܻ���̼���ƣ��ס��ҡ�����λͬѧ�ֱ�ȡ������ˮ���м��飮
��2011?�����ʼ죩���������ƹ����п��ܻ���̼���ƣ��ס��ҡ�����λͬѧ�ֱ�ȡ������ˮ���м��飮�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com