
��8�֣���ͼ���ף���A��B��C���ֹ������ʵ��ܽ������ͼ���ش��������⣺
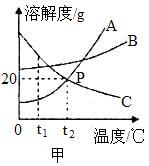
��1�������ϵ�P���ʾ������Ϊ______________________________��
��2��t2��ʱ����50gˮ�м���20gA���ʳ���ܽ⣬������Һ����������Һ��������Ϊ________________��
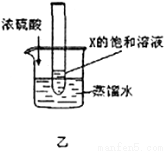
��3������ͼ���ң���ʾ���������µ�����ˮ����������Ũ���ᣬ��������Թ��ڵ���Һ�г��־��塣��XӦ����A��B��C���������е�______________��
��4��t1��ʱ����A��B��C�����ʵı�����Һ�ֱ����µ�t2����������Һ�����������������ɴ�С�Ĺ�ϵ��_________________��
��1�����¶�Ϊt2��ʱ��A��C�����ʵ��ܽ����ȡ� ��2��1:6��20:120�� ��3��C ��4��B>C>A
��������
�����������1�������ϵ�P����A��C�Ľ��㣬��Ӧ���¶�Ϊt2����������ʾ������Ϊ���¶�Ϊt2��ʱ��A��C�����ʵ��ܽ����ȡ�
��2��t2��ʱ��A���ܽ��Ϊ20g������t2��ʱ��100gˮ���ܽ�20gA�ɴﵽ���͡������50gˮ�м�������ܽ�10gA���ʣ�������Һ����������Һ��������Ϊ10g ����10g +50g��=1��6��
��3��Ũ�����ܽ���ˮʱ��ų��������ȡ��������ձ����¶Ȼ����ߡ��������Թ��ڵ���Һ�г��־�����˵��X�������¶�����ʱ����䱥����Һ�����������Կ�֪X���ܽ�Ȼ������¶ȵ����߶����ͣ���XΪC��
��4��t1�����µ�t2��ʱ��AB���ܽ�Ⱦ�������������塣��������Һ�е����������Ժ�t1��ʱ���ǵı�����Һ������������ȣ�����t1��ʱ�����ʵ��ܽ�Ƚ��м��㡣C������ʱ���ܽ�ȱ�С�����й����������������γɵ���Һ��Ϊ������Һ������Һ�����ʵ���������Ӧ��C������t2��ʱ�ܽ�Ƚ��м��㡣����ͼʾ��֪��t1��ʱB���ܽ�ȴ���t2��ʱC���ܽ�ȣ�t2��ʱC���ܽ�ȴ���t1��ʱA���ܽ�ȡ�����������Һ�����������������ɴ�С�Ĺ�ϵ��B>C>A��
���㣺�ܽ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014�ӱ�ʡ��ɽ��·�����п���ģ��ѧ�Ծ��������棩 ���ͣ�̽����
ʵ��С���о����ᡢ�������ơ����������������ʵĻ�ѧ���ʣ�������ͼ��ʾ��ʵ�顣��������ͼ������ҩƷ������һ������о���
��1��ʵ���ij�Թ���Ϊ��ɫ��Һ�����Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ���ij�Թ���Ϊ��ɫ��Һ�������м��������� ����Һ��Ϊ��ɫ���ɴ��ƶϣ����Թ������ʢ�е������� ��
��3��ʵ���ij�Թܵײ�����ɫ��״���������������м��������� ���������ʧ��
��4��ʵ���ij�Թܵĵײ��а�ɫ���壬���˺�����Һ�еμ�ϡ���ᣬ��ʼʱ����������һ��ʱ��������ݳ��֡��ɴ��ƶϣ����Թ������������Ӧ�Ļ�ѧ����Ϊ ��
��5��ʵ���ij�Թ���ֻ�õ���ɫ��Һ�������м���������̼������Һ��ֻ�������ݡ���ô���Թ������������Ӧ�Ļ�ѧ����Ϊ ��
��6��ʵ�����ʱ�����ǽ�ʵ���Һ����ͬһ��Һ���У��۲�����Dz������յķ�Һ���ܳ����ԡ�Ϊ����֤���룬��������ʵ����δ�漰������Ļ�ѧ���ʣ���ѡҩƷ����ʵ�飺ȡ������Һ��Ʒ���Թ��У������м���һ������ ���۲�����֤�����յķ�Һ�����ԡ�ʵ���Ϊ�˱����Һ��ɲ������ �����ǶԷ�Һ������ǡ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ��ѧ�ھ��꼶��һ�λ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ѧ�仯�Ľ�����С������ʡ����ɣ�����ѷ�Ӧ����ǡ������ʡ�������ԡ������ʡ��͡������ʡ������⣬��ȷ���ǣ� ��
A�� ��������ָ������û�е�����
B�� �����ʲ����ٱ����������
C�� �����ʺ;����ʵ����ʼ���;��������ȫ��ͬ
D�� �����ʵ�Ԫ�����������ʵ�Ԫ����ɿ϶���ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ�����н��п���ѧģ������Ծ��������棩 ���ͣ�ѡ����
����M�ڲ�ͬ�¶��µ��ܽ���������±���ʾ������˵����ȷ���ǣ� ��
�¶ȣ��棩 | 0 | 20 | 40 | 60 | 80 |
�ܽ�ȣ�g�� | 13.6 | 16.3 | 20.0 | 25.0 | 39.2 |
A��0��ʱ��15.6gM����120gˮ���γɱ�����Һ
B��20��ʱ��M�γɱ�����Һ����������������16.3%
C��80��ʱ��M�ı�����Һ139.2g������40�棬�����������������20.0g
D����Ҫ����300.0g20.0%��M�ı�����Һ����Ӧ�¶ȱ�����60��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ�����м������п���һ��ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ�������
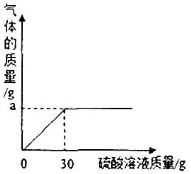
��ľ����һ��ũ�ҷʣ�������Ҫ�ɷ���̼��أ���������أ��Ȼ��صȡ���ѧ��ȤС��Ϊ�ⶨij��ľ����Ʒ�е���Ч�ɷ֣�ȡ50g��ľ�����ձ��У����ϵ���������Һ��������30g������Һʱ�����������ݲ�������ʱ�ձ��еIJ������������Ϊ77.8g���������ľ�ҵ������ɷֲ�����Ԫ���Ҳ����ᷴӦ��
�����ش�
��1����ľ�ҵ���Ҫ�ɷ��������������Ϊ____________����ᡢ����Ρ�����
��2����ͼ��ʾ��Ӧ�����зų����������������������Һ�Ĺ�ϵ���ߣ������ͼ����������a����ֵ��a=________________g��
��3�������ľ����Ʒ��̼��ص�������������Ҫ��д��������̣�
��4��ͨ������ʵ�飬��ø�50g��ľ���л��������������Ϊ8.7g���Ȼ��ص�����Ϊ1.49g�����50g��ľ����Ʒ�м�Ԫ�ص�����Ϊ__________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ�����м������п���һ��ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��AgNO3��Cu��NO3��2�Ļ����Һ�м���һ���������ۣ���ַ�Ӧ����ˣ��������м���ϡ���ᣬ�����ݲ��������������������ܵó��Ľ����ǣ� ��
A�� ��Һ��һ����Cu2+��Fe2+B�� ��Һ��һ����Ag+��Cu2+
C�� ��Һ��һ����Fe2+D�� ������һ����_____________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ�����м������п���һ��ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
С��ͬѧ�Բ��ֻ�ѧ֪ʶ�������£�������ȫ��ȷ��һ���ǣ� ��
A�� ���ʵķ��� | B�� ��ѧ�뻷�� |
����������ֻ����һ������ ���������к�����Ԫ�� ���������һ�����ж���Ԫ�� | ��pHС��7����ˮ�������� ������ӵ�µ����������������β�� �������ų����̳����γ�����Ⱦ���� |
C�� ����̼���á��Ĵ�ʩ | D�� ��ѧ�밲ȫ |
���������̭���ܺġ�����Ⱦ��ҵ �����ƺͿ�������Դ�����ͳ��Դ ������ʹ��һ�������Ϸ���� | ������Һ����Ƥ���ϣ������ô��������ϴ ����ȼ����ǰһ��Ҫ���鴿�� �������ľƾ�������ȼ�գ���ʪ���˸� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ��ѧ�����п��Գ�����ѧ�Ծ��������棩 ���ͣ�ѡ����
�±��г��ӷ�����ȷ���ǣ�������
ѡ�� | ���� | �������� | �����ʵķ��� |
A | �Ȼ��ƹ��� | ��ɳ | ��ˮ�ܽ⣬����ϴ�ӣ����� |
B | ������ | ̼��� | ��ˮ�ܽ⣬���� |
C | ������̼ | ˮ���� | ͨ��װ��Ũ�����ϴ��ƿ |
D | ����ͭ��Һ | ����������Һ | ����ͭ�ۣ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ�������п�һģ��ѧ�Ծ��������棩 ���ͣ�ѡ����
��ȥ���и������е��������ʣ����÷��������е��ǣ�������
ѡ�� | ���� | ���� | ��ȥ���ʵķ��� |
A | CO2 | CO | ͨ�����ȵ�����ͭ |
B | NaCl | ��ɳ | ��ˮ�ܽ⡢���ˡ����� |
C | NaOH | Na2CO3 | ��������ϡ���������ٲ������� |
D | HNO3��Һ | HCl | ������AgNO3��Һ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com