
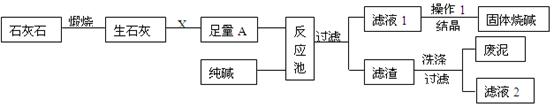
ПВНјКЗДі№¤і§ЙъІъЙХјоµДЦчТЄБчіМЎЈ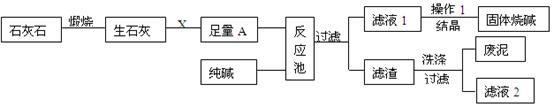
(1)XµД»ЇС§КЅОЄ ЎЈ
(2)ёГі§ЙъІъ№эіМЦРмСЙХБЛє¬МјЛбёЖ75%µДКЇ»ТКЇ12tЈ¬АнВЫЙПїЙЦЖµГСх»ЇёЖ tЎЈ
(3)ЧгБїµДAУлґїјоФЪ·ґУ¦іШЦР·ґУ¦µД»ЇС§·ЅіМКЅОЄ ЎЈ
(4)Ѕбѕ§µГµЅµД№ММеЙХјоЈ¬ѕІв¶Ёє¬УРЗвСх»ЇДЖµДЦКБї·ЦКэОЄ99.2%Ј¬»№є¬µДЙЩБїµДФУЦКЈ¬ФУЦККЗ Ј¬ФТтКЗ ЎЈ
(5)ВЛТє2ЦРµДИЬЦКµД»ЇС§КЅОЄ Ј¬ОЄБЛЅµµНЙъІъіЙ±ѕєН·АЦ№¶Ф»·ѕіФміЙОЫИѕЈ¬ДгµДЅЁТйКЗ ЎЈ
(1) H2O (2) 5.04t
(3)Ca(OH)2 + Na2CO3 = CaCO3Ўэ+ 2NaOH
(4)Na2CO3 NaOHИЬТєФЪ№эВЛЎўХф·ўЎўЅбѕ§№эіМЦРОьКХБЛїХЖшЦРµДCO2ЖшМе
(5)Ca(OH)2 Ѕ«ВЛТєCa(OH)2ЦШРВЧўИлµЅ·ґУ¦іШЦРС»·АыУГ
ЅвОцКФМв·ЦОцЈє(1)ёщѕЭЦКБїКШєг¶ЁВЙЈ¬»ЇС§·ґУ¦З°єуёчФЄЛШФЧУёцКэ·ґУ¦З°єуІ»±дЈ¬ФтXµД»ЇС§КЅОЄH2OЈ»(2)·ўЙъµД·ЅіМКЅОЄ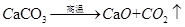 Ј¬ІОјУ·ґУ¦µДМјЛбёЖєНСх»ЇёЖµДЦКБї±ИОЄ100:56=25:14Ј¬ФтУРАнВЫЙПїЙЦЖµГСх»ЇёЖ
Ј¬ІОјУ·ґУ¦µДМјЛбёЖєНСх»ЇёЖµДЦКБї±ИОЄ100:56=25:14Ј¬ФтУРАнВЫЙПїЙЦЖµГСх»ЇёЖ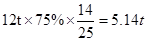 Ј»(3)AОЄЗвСх»ЇёЖЈ¬єНґїјо·ўЙъёґ·ЦЅв·ґУ¦Ј¬ЙъіЙЗвСх»ЇДЖєНМјЛбёЖЈ¬·ЅіМКЅОЄ
Ј»(3)AОЄЗвСх»ЇёЖЈ¬єНґїјо·ўЙъёґ·ЦЅв·ґУ¦Ј¬ЙъіЙЗвСх»ЇДЖєНМјЛбёЖЈ¬·ЅіМКЅОЄ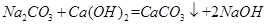 Ј»(4)є¬УРµДФУЦККЗМјЛбДЖЈ¬ТтОЄNaOHИЬТєФЪ№эВЛЎўХф·ўЎўЅбѕ§№эіМЦРОьКХБЛїХЖшЦРµДCO2ЖшМеЈ»(5)ВЛФьЦРє¬УРМјЛбёЖєНЗвСх»ЇёЖЈ¬ПґµУ№эВЛєуВЛТєЦРє¬УРЗвСх»ЇёЖЈ¬ОЄБЛЅµµНЙъІъіЙ±ѕєН·АЦ№ОЫИѕ»·ѕіЈ¬У¦Ѕ«ЗвСх»ЇёЖ»ШКХС»·АыУГЎЈ
Ј»(4)є¬УРµДФУЦККЗМјЛбДЖЈ¬ТтОЄNaOHИЬТєФЪ№эВЛЎўХф·ўЎўЅбѕ§№эіМЦРОьКХБЛїХЖшЦРµДCO2ЖшМеЈ»(5)ВЛФьЦРє¬УРМјЛбёЖєНЗвСх»ЇёЖЈ¬ПґµУ№эВЛєуВЛТєЦРє¬УРЗвСх»ЇёЖЈ¬ОЄБЛЅµµНЙъІъіЙ±ѕєН·АЦ№ОЫИѕ»·ѕіЈ¬У¦Ѕ«ЗвСх»ЇёЖ»ШКХС»·АыУГЎЈ
їјµгЈєЙъІъЙХјоµДБчіМ
µгЖАЈєґЛМвїјІмЦЄК¶µгЅПОЄД°ЙъЈ¬µ«ЧРПёЙуМвїЙЦЄїјІмµДЛјПлєН»щ±ѕЦЄК¶µг¶јКЗЦРїјТЄЗуµД»щ±ѕЦЄК¶Ј¬ґЛМвУРТ»¶ЁµДДС¶ИЈ¬ТЄ¶а¶БМбЈ¬¶аБЄПµМвёЙЎЈ



| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєіхЦР»ЇС§ АґФґЈє2013Дк№г¶«КЎ№гЦЭКР·¬Ш®ЗшЦРїјТ»ДЈ»ЇС§КФѕнЈЁЅвОц°жЈ© МвРНЈєМоїХМв
ПВНјКЗДі№¤і§ЙъІъЙХјоµДЦчТЄБчіМЎЈ
(1)XµД»ЇС§КЅОЄ ЎЈ
(2)ёГі§ЙъІъ№эіМЦРмСЙХБЛє¬МјЛбёЖ75%µДКЇ»ТКЇ12tЈ¬АнВЫЙПїЙЦЖµГСх»ЇёЖ tЎЈ
(3)ЧгБїµДAУлґїјоФЪ·ґУ¦іШЦР·ґУ¦µД»ЇС§·ЅіМКЅОЄ ЎЈ
(4)Ѕбѕ§µГµЅµД№ММеЙХјоЈ¬ѕІв¶Ёє¬УРЗвСх»ЇДЖµДЦКБї·ЦКэОЄ99.2%Ј¬»№є¬µДЙЩБїµДФУЦКЈ¬ФУЦККЗ Ј¬ФТтКЗ ЎЈ
(5)ВЛТє2ЦРµДИЬЦКµД»ЇС§КЅОЄ Ј¬ОЄБЛЅµµНЙъІъіЙ±ѕєН·АЦ№¶Ф»·ѕіФміЙОЫИѕЈ¬ДгµДЅЁТйКЗ ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєіхЦР»ЇС§ АґФґЈє МвРНЈє
ПВНјКЗДі№¤і§ЙъІъЙХјоµДЦчТЄБчіМЎЈ

(1)XµД»ЇС§КЅОЄ ЎЈ
(2)ёГі§ЙъІъ№эіМЦРмСЙХБЛє¬МјЛбёЖ75%µДКЇ»ТКЇ12tЈ¬АнВЫЙПїЙЦЖµГСх»ЇёЖ tЎЈ
(3)ЧгБїµДAУлґїјоФЪ·ґУ¦іШЦР·ґУ¦µД»ЇС§·ЅіМКЅОЄ ЎЈ
(4)Ѕбѕ§µГµЅµД№ММеЙХјоЈ¬ѕІв¶Ёє¬УРЗвСх»ЇДЖµДЦКБї·ЦКэОЄ99.2%Ј¬»№є¬µДЙЩБїµДФУЦКЈ¬ФУЦККЗ Ј¬ФТтКЗ ЎЈ
(5)ВЛТє2ЦРµДИЬЦКµД»ЇС§КЅОЄ Ј¬ОЄБЛЅµµНЙъІъіЙ±ѕєН·АЦ№¶Ф»·ѕіФміЙОЫИѕЈ¬ДгµДЅЁТйКЗ ЎЈ
Ійїґґр°ёєНЅвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com