
 %��������е�Ԫ�ص�����������
%��������е�Ԫ�ص�����������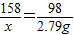
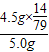 ×100%=15.9%
×100%=15.9%


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2007�����ʡ�Ƹ�����У������ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008���п���ѧ�ۺ�ѵ���Ծ���2���������棩 ���ͣ�ѡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʡ��ͷ�г������п���ѧģ���Ծ��������棩 ���ͣ������
| ����Һ | NaCl | Na2CO3 | BaCl2 |
| pH | ����7 | ����7 | ����7 |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��1��ȡ��������Һ���Թ��У�����Һ�еμ��������Ȼ�����Һ���������� | ˵��ԭ����Һ�У�һ�����е�������̼���ƣ� | |
| ��2�����裨1����ַ�Ӧ�����Һ�еμӣ� | ˵��ԭ����Һ�У���һ�����У� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008�걱���г������п���ѧ��ģ�Ծ��������棩 ���ͣ�ѡ����
 ��2008?��������ģ����ͼ��ij�Լ�ƿ���Ѿ����ֱ������ʴ�ı�ǩ��С����̽����ɷ֣���ȡһ֧�ྻ���Թܣ����������Լ�ƿ�еĹ��壬�����������ᣬ����ɫ��ζ���ݲ�������������ʹ����ʯ��ˮ����ǣ���ù��壨 ��
��2008?��������ģ����ͼ��ij�Լ�ƿ���Ѿ����ֱ������ʴ�ı�ǩ��С����̽����ɷ֣���ȡһ֧�ྻ���Թܣ����������Լ�ƿ�еĹ��壬�����������ᣬ����ɫ��ζ���ݲ�������������ʹ����ʯ��ˮ����ǣ���ù��壨 ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2007�꽭��ʡ��ͨ���綫��Ԭׯ��ѧ��ǰ������ѧ�Ծ��������棩 ���ͣ�ѡ����
 ��2008?��������ģ����ͼ��ij�Լ�ƿ���Ѿ����ֱ������ʴ�ı�ǩ��С����̽����ɷ֣���ȡһ֧�ྻ���Թܣ����������Լ�ƿ�еĹ��壬�����������ᣬ����ɫ��ζ���ݲ�������������ʹ����ʯ��ˮ����ǣ���ù��壨 ��
��2008?��������ģ����ͼ��ij�Լ�ƿ���Ѿ����ֱ������ʴ�ı�ǩ��С����̽����ɷ֣���ȡһ֧�ྻ���Թܣ����������Լ�ƿ�еĹ��壬�����������ᣬ����ɫ��ζ���ݲ�������������ʹ����ʯ��ˮ����ǣ���ù��壨 ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com