
���� ʵ����Ƶ�һ����ȷ����������������Ԫ�ص��������ڶ�����ȷ����Ԫ�ص��������������ݸ��Ե��������Ը������ԭ���������㻯ѧʽ��ab����ֵȷ�����Ļ�����Ļ�ѧʽ��
��� �⣺��1����Һ�����������������ɵĶ�����̼���������������ɵĶ�����̼������Ϊ17.6g��������������Ԫ�ص�������һ��������������������������������е���Ԫ�ص�����Ϊ$\frac{16��2}{12+16��2}$��$100%��17.6g��\frac{1}{2}$=6.4g��
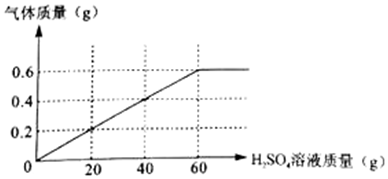
��2������ͼ��֪������Ӧ������������Ϊ0.6g��
������ϡ������Һ����������Ϊx����������Ϊy
Fe+H2SO4=FeSO4+H2��
56 98 2
y x 0.6g
$\frac{56}{y}$=$\frac{98}{x}$=$\frac{2}{0.6g}$
x=29.4g
y=16.8g
����ϡ������Һ��������������Ϊ��$\frac{29.4g}{60g}$��100%=49%
��3����ԭ�ӵĸ�������ԭ�ӵĸ�����Ϊa��b=$\frac{16.8g}{56}$��$\frac{6.4g}{16}$=3��4��
�𣺣�1������ I���������������Ϊ 17.6g��
��2������ II����ϡ������Һ��������������Ϊ49%��
��3�������������� I��ּ��Ⱥ�ʣ���������Ϊ22.6g��������������ﻯѧʽ�У�a��b�����������a��b=3��4��
���� ���ݻ�ѧ����ʽ����ʱ����һҪ��ȷ��д��ѧ����ʽ���ڶ�Ҫʹ����ȷ�����ݣ������������Ҫ������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѩ���� | B�� | �Ƹ����� | C�� | ���Ͻ��� | D�� | ��ˮ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʯ��ˮ | B�� | ϡ���� | C�� | ϡ���� | D�� | ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
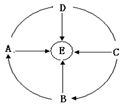 A��E�dz��г��������ʣ����ǵ�ת����ϵ��ͼ��ʾ��ͼ�С���������ת����ϵ����--����ʾ���Ӧ����B�����ڸ�������������C��E���������E�����庬���������ʣ���ش��������⣺
A��E�dz��г��������ʣ����ǵ�ת����ϵ��ͼ��ʾ��ͼ�С���������ת����ϵ����--����ʾ���Ӧ����B�����ڸ�������������C��E���������E�����庬���������ʣ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����θҺ�������ᣬ�ɰ������� | |
| B�� | ú��ʯ�ͺ���Ȼ�����ǿ�������Դ | |
| C�� | ����ȱ����Ԫ������ɼ�״���״� | |
| D�� | �����ά���ض���Ϊ�����ṩ��������Ҫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com