
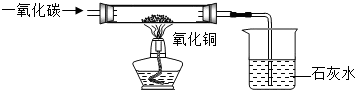
 ���к����ʵ�����ͭ��Ʒ�����ʲ��μӷ�Ӧ����Ϊ�˲ⶨ����Ʒ������ͭ������������ijͬѧ��ȡ12.5g��Ʒ��������ͼ��ʾ��װ�ý���ʵ�飬�õ������������ݣ�
���к����ʵ�����ͭ��Ʒ�����ʲ��μӷ�Ӧ����Ϊ�˲ⶨ����Ʒ������ͭ������������ijͬѧ��ȡ12.5g��Ʒ��������ͼ��ʾ��װ�ý���ʵ�飬�õ������������ݣ�| ��Ӧǰ | ����ͭ��ȫ��Ӧ�� | |
| ��һ�� | �ձ��ͳ���ʯ��ˮ��������180.0g | �ձ����ձ������ʵ�������184.3g |
| �ڶ��� | �����ܺ�����ͭ��Ʒ����������44.5g | �����ܺ������ʵ���������42.9g |
 Cu+CO2��CO2+Ca��OH��2=CaCO3��+H2O��
Cu+CO2��CO2+Ca��OH��2=CaCO3��+H2O�� Cu+CO2 ��m
Cu+CO2 ��m ��100%=64%
��100%=64% Cu+CO2�����
Cu+CO2�����


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��Ӧǰ | ����ͭ����ȫ��ԭ�� |  | |
| A�� | �����ܺ�����ͭ��Ʒ����Ϊm1g | �����ܺ������ʵ�����Ϊm2g | |
| B�� | �ձ��ͳ���ʯ��ˮ������Ϊm3g | �ձ����ձ������ʵ�����Ϊm1g |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2005?������һģ�����к����ʵ�����ͭ��Ʒ�����ʲ��μӷ�Ӧ����Ϊ�˲ⶨ����Ʒ������ͭ������������ijͬѧ��ȡ12.5g��Ʒ��������ͼ��ʾ��װ�ý���ʵ�飬�õ������������ݣ�
��2005?������һģ�����к����ʵ�����ͭ��Ʒ�����ʲ��μӷ�Ӧ����Ϊ�˲ⶨ����Ʒ������ͭ������������ijͬѧ��ȡ12.5g��Ʒ��������ͼ��ʾ��װ�ý���ʵ�飬�õ������������ݣ�| ��Ӧǰ | ����ͭ��ȫ��Ӧ�� | |
| ��һ�� | �ձ��ͳ���ʯ��ˮ��������180.0g | �ձ����ձ������ʵ�������184.3g |
| �ڶ��� | �����ܺ�����ͭ��Ʒ����������44.5g | �����ܺ������ʵ���������42.9g |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
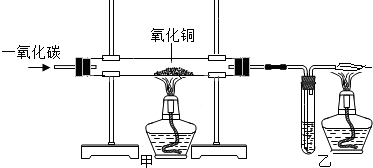
| ��Ӧǰ | ����ͭ��ȫ��Ӧ�� | |
| ���� | ϴ��ƿ��ʯ��ˮ������Ϊ185g | ϴ��ƿ��ƿ�����ʵ�������Ϊ187g |
| ���� | �����ܺ�����ͭ��Ʒ������Ϊ57.9g | �����ܺͲ����������ʵ�����Ϊ56.1g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����������ģ ���ͣ��ʴ���
| ��Ӧǰ | ����ͭ����ȫ��ԭ�� | 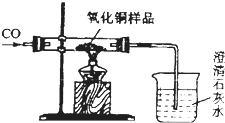 | |
| A�� | �����ܺ�����ͭ��Ʒ����Ϊm1g | �����ܺ������ʵ�����Ϊm2g | |
| B�� | �ձ��ͳ���ʯ��ˮ������Ϊm3g | �ձ����ձ������ʵ�����Ϊm1g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008���Ϻ����������п���ѧ��ģ�Ծ��������棩 ���ͣ������
| ��Ӧǰ | ����ͭ����ȫ��ԭ�� | 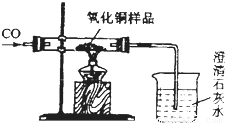 | |
| A�� | �����ܺ�����ͭ��Ʒ����Ϊm1g | �����ܺ������ʵ�����Ϊm2g | |
| B�� | �ձ��ͳ���ʯ��ˮ������Ϊm3g | �ձ����ձ������ʵ�����Ϊm1g |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com