
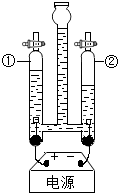
 Ė®ŗĶČÜŅŗŌŚÉśĆü»ī¶ÆŗĶÉś²ś”¢Éś»īÖŠĘš×ÅŹ®·ÖÖŲŅŖµÄ×÷ÓĆ£®
Ė®ŗĶČÜŅŗŌŚÉśĆü»ī¶ÆŗĶÉś²ś”¢Éś»īÖŠĘš×ÅŹ®·ÖÖŲŅŖµÄ×÷ÓĆ£®| ĪĀ¶Č£Ø”ę£© | 0 | 20 | 40 | 60 | 80 | 100 | |
| Čܽā¶Č £Øg£© | NaOH | 31 | 91 | 111 | 129 | 313 | 336 |
| Ca£ØOH£©2 | 0.19 | 0.17 | 0.14 | 0.12 | 0.09 | 0.08 | |


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢”°Ģ½¾æĢ¼ĖįÄʵĊŌÖŹ”±ŹµŃéÖŠ£¬²ā¶ØČÜŅŗpHŹ±Ó¦ĻČ½«ŹŌÖ½ŹŖČó |
| B”¢”°“ÖŃĪµÄĢį“æ”±ŹµŃéÖŠ£¬¹żĀĖŹ±½«Šü×ĒŅŗÖ±½Óµ¹ČėĀ©¶·Ąļ |
| C”¢”°ÅäÖĘŅ»¶ØČÜÖŹÖŹĮæ·ÖŹżµÄČÜŅŗ”±ŹµŃéÖŠ£¬¶ąÓąµÄŅ©Ę·Ó¦·Å»ŲŌĘæ |
| D”¢”°øßĆĢĖį¼ŲÖĘČ”ŃõĘų”±ŹµŃéÖŠ£¬ŹÕ¼ÆŗĆĘųĢåŗóÓ¦ĻČ½«µ¼¹ÜŅĘ³öĖ®²ŪŌŁĶ£Ö¹¼ÓČČ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢ÓĆ×ĘÉÕĪÅĘųĪ¶Ēų·ÖŃņČŽŗĶĆŽĻß |
| B”¢ÓĆĻ”ŃĪĖįĒų·ÖĢś·ŪŗĶŃõ»ÆĶ |
| C”¢ÓĆ·ÓĢŖČÜŅŗĒų·ÖĻ”ŃĪĖįŗĶŹ³ŃĪĖ® |
| D”¢ĶعżĘ·³¢Ēų·Ö³ų·æÖŠµÄŹ³ŃĪŗĶŹüĢĒ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢ĢģČ»ĘųČ¼ÉÕ |
| B”¢ĢśŌŚ³±ŹŖµÄ»·¾³ÖŠÉśŠā |
| C”¢ŹÆÓĶ·ÖĮóµĆµ½ĘūÓĶ |
| D”¢ĆŗøÉĮóµĆµ½½¹Ģæ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com