
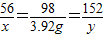
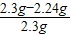 ×100%”Ö2.6%£¬ĖłŅŌøĆѳʷĪŖÉśĢś£»
×100%”Ö2.6%£¬ĖłŅŌøĆѳʷĪŖÉśĢś£»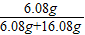 ×100%=27.4%£»
×100%=27.4%£»

 ½ņĒŽĢÓż¼ĘĖ抔דŌŖĻµĮŠ“š°ø
½ņĒŽĢÓż¼ĘĖ抔דŌŖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗĖÕÖŻĻą³ĒĒų2006£2007ѧğ¶ČµŚŅ»Ń§ĘŚ³õȿğ¼¶ĘŚÄ©²āŹŌ¾ķ(»Æѧ) ĢāŠĶ£ŗ059
ijĶ¬Ń§Ń§Ļ°ĮĖĢśŗĻ½šŗóŗÜøŠŠĖȤ£¬Ėū“Ó¼ŅÖŠČ”Ą“ĮĖĢśÖĘʷѳʷ£¬¾³żŠā“ņÄ„“¦Ąķŗó£¬ŌŚĄĻŹ¦µÄ°ļÖśĻĀ£¬ŌŚŹµŃéŹŅ½ųŠŠĮĖĢśµÄÖŹĮæ·ÖŹż²ā¶Ø£¬ĖūČ”2£®3æĖѳʷ£¬·ÅŌŚÉÕ±ÖŠ£¬Č»ŗóµĪ¼Ó19.6£„µÄĻ”ĮņĖįµ½²»ŌŁ²śÉśĘųÅŻĪŖÖ¹£¬¹²¼ĘĻūŗÄĻ”ĮņĖį20æĖ£¬øĆĶ¬Ń§×÷ĮĖ¼ĘĖćŗĶĢ½¾æ£®
(1)øĆĢśÖĘĘ·ŹĒÉśĢś»¹ŹĒøÖ£æ(ÉśĢśµÄŗ¬Ģ¼ĮæĪŖ2£„”«4.3£„£¬øÖµÄŗ¬Ģ¼ĮæĪŖ0.03£„”«2£„)
(2)·“Ó¦ŗóĖłµĆČÜŅŗČÜÖŹÖŹĮæ·ÖŹżĪŖ¶ąÉŁ£æ
(3)øĆĶ¬Ń§×öĶźŹµŃéŗó£¬Ć»ÓŠĻóĶł³£Ņ»Ńł½«·ĻŅŗµ¹ŌŚ·ĻŅŗ±ÖŠ£¬ĖūČĻĪŖ·ĻŅŗ²»·Ļ£¬×÷ĪŖĶ¬°é£¬ÄćŹĒ·ńŌŽĶ¬ĖūµÄ¹Ūµć£æĪŖŹ²Ć“£æ
(4)ČēŗĪ»ŲŹÕĖłµĆČÜŅŗÖŠµÄČÜÖŹ£æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com