
| ŹµŃéŠņŗÅ | ¼× | ŅŅ | ±ū |
| ŗĻ½šÖŹĮæ£ØæĖ£© | 0.51 | 0.70 | 0.90 |
| ĘųĢåÖŹĮæ£ØæĖ£© | 0.05 | 0.06 | 0.06 |
·ÖĪö £Ø1£©Óɼ×ŅŅÖŠµÄŹż¾ŻæÉÖŖ£¬ŗĻ½šµÄÖŹĮæŌö“óµÄ±¶ŹżĪŖ£ŗ0.70g”Ā0.51g=1.510±¶£¬ĘųĢåÖŹĮæŌö“óµÄ±¶ŹżĪŖ£ŗ0.06g”Ā0.05g=1.2±¶£¬ÓÉ“ĖæÉÖŖ£¬¼×ÖŠŃĪĖį¹żĮ森ÓÉŅŅ±ūÖŠµÄŹż¾ŻæÉÖŖ£¬Ėę×ÅŗĻ½šÖŹĮæµÄŌö¼Ó£¬Éś³ÉĘųĢåµÄÖŹĮæ²»±ä£¬ĖµĆ÷ŅŅÖŠŃĪĖį²»×ćĮ森
£Ø2£©øł¾Ż¼×ÖŠŹż¾ŻæÉŅŌĒó³öŗĻ½šÖŠĆ¾”¢ĀĮµÄÖŹĮ森
£Ø3£©ÓɱķÖŠŹż¾ŻæÉÖŖ£¬30æĖŃĪĖįČÜŅŗ×ī¶ą²śÉś0.06gĒāĘų£¬øł¾ŻĒāĘųÖŹĮ漓æɽā“š£»
½ā“š ½ā£ŗ£Ø1£©Óɼ×ŅŅÖŠµÄŹż¾ŻæÉÖŖ£¬ŗĻ½šµÄÖŹĮæŌö“óµÄ±¶ŹżĪŖ£ŗ0.70g”Ā0.51g=1.510±¶£¬ĘųĢåÖŹĮæŌö“óµÄ±¶ŹżĪŖ£ŗ0.06g”Ā0.05g=1.2±¶£¬ÓÉ“ĖæÉÖŖ£¬¼×ÖŠŃĪĖį¹żĮ森Ėę×ÅŗĻ½šÖŹĮæµÄŌö¼Ó£¬Éś³ÉĘųĢåµÄÖŹĮæ²»±ä£¬ĖµĆ÷ŅŅÖŠŃĪĖį²»×ćĮ森¹ŹĢī£ŗ²»×ćĮ森
£Ø2£©½āÉčĀĮµÄÖŹĮæĪŖX£¬Éś³ÉĒāĘųµÄÖŹĮæĪŖY£®
Mg+2HCl=MgCl2+H2”ü£¬2Al+6HClØT2AlCl3+3H2”ü£®
24 2 54 6
0.51g-X 0.05g-Y X Y
$\frac{24}{2}=\frac{0.51g-X}{0.05g-Y}$¢Ł
$\frac{54}{X}=\frac{6}{Y}$¢Ś
ÓÉ¢Ł¢ŚæÉµĆ£ŗX=0.27g£¬Y=0.03g£¬ŌņĆ¾µÄÖŹĮæĪŖ£ŗ0.51g-0.27g=0.24g£¬Ōņ ŗĻ½šÖŠĆ¾”¢ĀĮµÄÖŹĮæ±ČĪŖ£ŗ0.24g£ŗ0.27g=8£ŗ9£®
“š£ŗŗĻ½šÖŠĆ¾”¢ĀĮµÄÖŹĮæ±Č8£ŗ9£®
£Ø3£©ÓÉ·ÖĪöæÉÖŖ£¬30æĖŃĪĖįČÜŅŗ×ī¶ą²śÉś0.06gĒāĘų£»
Éč30æĖŃĪĖįČÜŅŗČÜÖŹÖŹĮæĪŖx£¬
2HCl”«H2”ü£¬
73 2
x 0.06g
$\frac{73}{x}=\frac{2}{0.06g}$
x=2.19g£»
ŃĪĖįµÄČÜÖŹÖŹĮæ·ÖŹż£ŗ$\frac{2.19g}{30g}$”Į100%=7.3%£»
“š£ŗŃĪĖįµÄČÜÖŹÖŹĮæ·ÖŹżĪŖ7.3%£®
µćĘĄ ±¾ĢāÖ÷ŅŖæ¼²é¼ĘĖć·½ĆęµÄÖŖŹ¶£¬½ųŠŠ¼ĘĖ揱ŅŖ°Ń»Æѧ·½³ĢŹ½ŗĶĻą¹ŲĮæŅ»Ņ»¶ŌӦʚĄ“½ųŠŠ


 ŹĄ¼Ķ°ŁĶØĘŚÄ©½š¾ķĻµĮŠ“š°ø
ŹĄ¼Ķ°ŁĶØĘŚÄ©½š¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
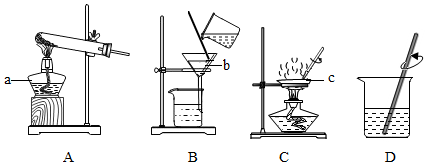
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ʶŃŖ | B£® | ¼×דĻŁÖדó | C£® | ŲžŁĶ²” | D£® | Ņ¹Ć¤Ö¢ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
 ČĖĢåĶعż·ĪÓėĶā½ē½ųŠŠĘųĢå½»»»£¬ĪüČėæÕĘųÖŠµÄŃõĘų£¬Åųö¶žŃõ»ÆĢ¼ŗĶĖ®ÕōĘų£®µ«ČĖĢåÅųöµÄ¶žŃõ»ÆĢ¼¾æ¾¹ŹĒæÕĘųÖŠŌÓŠµÄ£¬»¹ŹĒČĖĢå“śŠ»µÄ×īÖÕ²śĪļ£æĪŖĮĖÖ¤ŹµÕāøöĪŹĢā£¬ÓŠČĖ²ÉÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆ½ųŠŠŹµŃé£ŗ
ČĖĢåĶعż·ĪÓėĶā½ē½ųŠŠĘųĢå½»»»£¬ĪüČėæÕĘųÖŠµÄŃõĘų£¬Åųö¶žŃõ»ÆĢ¼ŗĶĖ®ÕōĘų£®µ«ČĖĢåÅųöµÄ¶žŃõ»ÆĢ¼¾æ¾¹ŹĒæÕĘųÖŠŌÓŠµÄ£¬»¹ŹĒČĖĢå“śŠ»µÄ×īÖÕ²śĪļ£æĪŖĮĖÖ¤ŹµÕāøöĪŹĢā£¬ÓŠČĖ²ÉÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆ½ųŠŠŹµŃé£ŗ²éæ““š°øŗĶ½āĪö>>
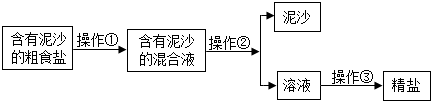
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
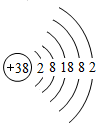 ¾Ż”¶×ŌČ»”·ŌÓÖ¾±ØµĄ£¬æĘѧ¼Ņ×ī½üŃŠÖʵÄŅ»ÖÖŅŌļČŌ×Ó×öÖÓ°ŚµÄÖÓŹĒŹĄ½ēÉĻ×ī¾«Č·µÄÖÓ£®ļČŌŖĖŲ£ØŌŖĖŲ·ūŗÅĪŖSr£©Ō×Ó½į¹¹Ź¾ŅāĶ¼ČēĶ¼ĖłŹ¾£®
¾Ż”¶×ŌČ»”·ŌÓÖ¾±ØµĄ£¬æĘѧ¼Ņ×ī½üŃŠÖʵÄŅ»ÖÖŅŌļČŌ×Ó×öÖÓ°ŚµÄÖÓŹĒŹĄ½ēÉĻ×ī¾«Č·µÄÖÓ£®ļČŌŖĖŲ£ØŌŖĖŲ·ūŗÅĪŖSr£©Ō×Ó½į¹¹Ź¾ŅāĶ¼ČēĶ¼ĖłŹ¾£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ĪŖĮĖ²ā¶ØæÕĘųÖŠŃõĘųµÄĢå»ż·ÖŹż£¬°“ČēĶ¼×°ÖĆ½ųŠŠŹµŃé£ŗ
ĪŖĮĖ²ā¶ØæÕĘųÖŠŃõĘųµÄĢå»ż·ÖŹż£¬°“ČēĶ¼×°ÖĆ½ųŠŠŹµŃé£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com