
���� ��1�����ݱ�������ӵĹ��ɽ��з������
��2��������Է�������Ϊ���ɷ��ӵĸ�ԭ�ӵ����ԭ������֮�ͣ���������Ԫ�ص���������=$\frac{���ԭ��������ԭ�Ӹ���}{��Է�������}$��100%�����з������
��3�����ݵ����ʵ��������������е�Ԫ�ص�ƽ����������=�̷��е�Ԫ�ص��������м����жϼ��ɣ�
��� �⣺��1��1���������������3��̼ԭ�ӡ�7����ԭ�ӡ�2����ԭ�Ӻ�1��ԭ�ӹ��ɵģ�������������C��H��O��N��ԭ�Ӹ�����Ϊ3��7��2��l��
��2�����������Է���������12��3+1��7+16��2+14=89����Ԫ�ص���������Ϊ$\frac{14}{89}$��100%��15.7%��
��3���ϸ��̷�ÿ100g�к�������Լ18g���������е�Ԫ�ص�ƽ����������Ϊ16%����100g�ϸ��̷��е�Ԫ�ص�������18g��16%=2.88g��2g����Ϊ���ϸ��̷ۣ�
�ʴ�Ϊ����1��3��7��2��l����2��15.7%����3�����ϸ��̷ۣ�
���� �����ѶȲ�����ͬѧ�ǽ������Ϣ��������û�ѧʽ�ĺ������йؼ�����з������⡢��������������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
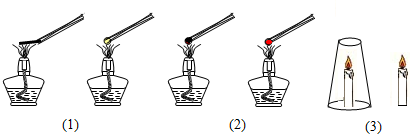
| ʵ�鲽�� | �����ͼ | ʵ������ | ʵ����� |
| ��1�� | ȼ�������ʵĹ�ϵ | ��ȼ�����ȼ�� | |
| ��2�� | ȼ�����¶ȵĹ�ϵ | ûպˮ��С����ȼ�� պˮ��С���Ų�ȼ�� | |
| ��3�� | ������������Ϩ���� ��������������ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ե��֭ | B�� | ʳ�︯�� | C�� | �������Ż� | D�� | �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڢۢ� | B�� | �ڢܢ� | C�� | �ڢ� | D�� | �٢ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ˮ�������������ŷ� | |
| B�� | ��ǿ��ҵ��ˮ���ŷż�أ���ִ���ŷ� | |
| C�� | ����ʩ��ũҩ�����ʣ��Լ���ˮ����Ⱦ | |
| D�� | ����ϴ�·ۿ�����ʹ�ã���������ˮ��Ⱦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��˿��������ȼ�� | B�� | ����������ȼ�� | ||
| C�� | �����ڿ�����ȼ�� | D�� | ľ̿�ڿ�����ȼ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com