
��������װ�ú�������ʾ��ͼ���ش���������.

��1�����Ϊb������������_______________��
��2������ʵ�����У���Ca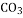 ��2HCl��Ca
��2HCl��Ca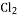 ��
��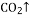 ��
��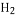 O����ȡ
O����ȡ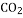 �����������ſ������ռ�������̼����װ���������ȡ���ռ�����ѡ����ͼ��a��b��c��h��j��n֮�Ӧѡ���������_______��
�����������ſ������ռ�������̼����װ���������ȡ���ռ�����ѡ����ͼ��a��b��c��h��j��n֮�Ӧѡ���������_______��
������100gCa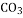 ��ȫ��Ӧ����ȡ
��ȫ��Ӧ����ȡ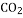 ________g.
________g.
��������֤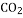 ����ʱ����
����ʱ����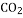 ͨ����ɫʯ����Һ����Һ��죬��ͨ�����ʯ��ˮ��ʼ��δ���ǣ��ݴ��Ʋ���ܵ�ԭ����______________________.
ͨ����ɫʯ����Һ����Һ��죬��ͨ�����ʯ��ˮ��ʼ��δ���ǣ��ݴ��Ʋ���ܵ�ԭ����______________________.
��3������ʵ�����ø��������ȡ��������ѡ����ͼ�п��õIJ��������⣬���貹��IJ���������_________���йصĻ�ѧ��Ӧ����ʽΪ_____________
 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ2019����꼶��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
ʵ��С��������ͼ��ʾװ�ò����������������������ȡ�óɹ���

[��������]�����Ż��40�档
[�������]�������Լռ����������Ķ��٣�
[ʵ����]��ƿ�ڿ������Ϊ200mL��ע������ˮ�����Ϊ50mL����װ�����������á�
[ʵ��̽��]װ��ҩƷ����ͼ��ʾ���Ӻ��������н����ɼС��Ƚ���ƿ�ײ�������ˮ�У����ܿ챻��ȼ��Ȼ����ƿ����ˮ��ȡ����
[�������]
��1������ƿ�ײ�������ˮ�У����ױ���ȼ��˵��ȼ�ղ���ȱ�ٵ�һ������_______________������֮�⣬ȼ�ջ���Ҫ��������_________________��_____________________��
��2������ȼ�յ�������_____________________��д������ȼ�յĻ�ѧ����ʽ��_____________________ ��������Ӧ������____________________��
��3�������İ�������ƿ��δ��ȫ��ȼ�գ�˵��ƿ��ʣ������______ȼ�գ��֧�֡���֧�֡�����
��4��������ʵ������У��ɹ۲쵽����ı仯��__________________��
��5��������Ϩ����ƿ��ȴ�����º��ɼУ����ɹ۲쵽�������ǣ���ע�����е�ˮ�Զ�����������ڵ�ע�����е�ˮ��ʣԼ10mLʱֹͣ������������Щ��������ԭ���ǣ�_______________��_________________��
[�ó�����]����Լռ�����������1/5 ��
[��չӦ��]
��6����ͬѧ�����������Ӧǰ��������װ�õ�����Ϊm�ˣ���Ӧ�������Ϩ����ƿ��ȴ�����£�������ƿ��ڵ�ˮ���ٴγ�������װ�õ�����Ϊ_________m��(����ڡ��������ڡ����� С�ڡ�)���жϵ�������_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ʡ�������е�����2019����꼶��ѧ���п�һģ��ѧ�Ծ� ���ͣ���ѡ��
�����������屻��ˮ���ջ����ɾ綾��ƫ���ᣬ�й�ƫ�����������ȷ���ǣ�������

A. ƫ�������⡢����������ԭ�ӹ���
B. ƫ�����������Ԫ�ص������������
C. ƫ�����������������һ�����ڵ�������
D. ƫ�������⡢�ס�������ԭ�ӵ�������Ϊ3��1��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2019����꼶��ѧ��3�½��Բ��Ի�ѧ�Ծ� ���ͣ���ѡ��
���й��ɺ��ܽ���ȫ��ȷ��һ����
A����ȫ����ʶ | B���á���ѧ���۹���� |
�ٵ�ȼ��ȼ������ǰһ��Ҫ�鴿 ��ú��й©Ӧ������������ͨ�� ��Ƥ��մ������������Һ�����ý϶�ˮ��ϴ | ����ˮ����ǽ����¶�����ȼ����Ż������ ���������С�մ��������ˮ ����ʯ�ҿ��Ը����������� |
C����ѧ�뽡�� | D���Գ��Ӻͼ������ʶ |
��ù��Ĵ���ϴ����������ʳ�� �����ü�ȩˮ��Һ����ˮ��Ʒ���� ������ȱ���ᵼ��ƶѪ | �����ȵ�CuO��ȥCO������CO2 �ڴ�Ũ�����ƿ���γɰ��� ����ȼ�յķ���������ë�͵��� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2019����꼶��ѧ��3�½��Բ��Ի�ѧ�Ծ� ���ͣ���ѡ��
���л�ѧ�����ʾ��ȷ����
A. ��������FeO B. �����ƵĻ�ѧʽ��Na2SO4
C. �����۵�þԪ�أ�Mg+2 D. ������Ne2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�����о��¸�����2019����꼶��ѧ��ģ�⿼�Ի�ѧ�Ծ� ���ͣ������
��ش�������ˮ�йص�����
��1�����ˮ����________�����鸺������������
��2���ճ������У�����__________�ķ�������ˮ��Ӳ�ȣ�
��3����ȥˮ����ɳ�����������ʣ����õIJ�����__________��
��4��ũҵ���ֽ���ֲ��ʱ��������ˮ�����Ϊ��ࡢ�ι��Ŀ����___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�����о��¸�����2019����꼶��ѧ��ģ�⿼�Ի�ѧ�Ծ� ���ͣ���ѡ��
��Ҷ�к��еIJ谱�ữѧʽΪ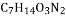 ������˵����ȷ���ǣ� ��
������˵����ȷ���ǣ� ��
A. �谱����̼���⡢������4��Ԫ�����
B. һ���谱������к���һ��������
C. �谱�����Է�������Ϊ174
D. �谱����̼Ԫ�غ���Ԫ�ص�������Ϊ7:14
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�����ɹ����������ͺ�����2019����꼶��ѧ���п�һģ��ѧ�Ծ� ���ͣ���ѡ��
���ж���ʵ�Ľ��Ͳ���ȷ����
ѡ�� | ��ʵ | ���� |
A | 6000L�����ڼ�ѹ����¿�װ���ݻ�Ϊ40L�ĸ�ƿ�� | ��ѹʱ�������Ӽ�����С |
B | ���ᡢϡ����Ļ�ѧ�������� | ���ᡢϡ�����ж����������� |
C | ���ʯ��ʯī���������ʴ��������Բ��� | ���ǵ�̼ԭ�����з�ʽ��ͬ |
D | H2�������ܵ�ȼ�� | H2ȼ������H2Oû����Ⱦ |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2019����꼶��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ���ѡ��
�Ŵ��������鶾����ԭ���ǣ�4Ag+2H2S+O2=2X+2H2O��X�Ļ�ѧʽΪ
A. AgS B.  C.
C.  D.
D. 
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com