
| æÕĘų | NH3 | CH4 | HCl | |
| ĆÜ¶Č£Øg/L£© | 1.293 | 0.771 | 0.717 | 1.629 |
| ČܽāŠŌ | - | ¼«Ņ×ČÜ | ¼«ÄŃČÜ | ¼«Ņ×ČÜ |


 æŚĖćĢāæؼÓÓ¦ÓĆĢā¼ÆѵĻµĮŠ“š°ø
æŚĖćĢāæؼÓÓ¦ÓĆĢā¼ÆѵĻµĮŠ“š°ø ×ŪŗĻ×Ō²āĻµĮŠ“š°ø
×ŪŗĻ×Ō²āĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ±ķÖŠĮŠ³öĮĖæÕĘų”¢NH3£Ø°±Ęų£©”¢CH4”¢HClĖÄÖÖĘųĢåŌŚ±ź×¼×“æöĻĀµÄĆܶČŗĶČܽāŠŌ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
±ķÖŠĮŠ³öĮĖæÕĘų”¢NH3£Ø°±Ęų£©”¢CH4”¢HClĖÄÖÖĘųĢåŌŚ±ź×¼×“æöĻĀµÄĆܶČŗĶČܽāŠŌ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| æÕĘų | NH3 | CH4 | HCl | |
| ĆÜ¶Č£Øg/L£© | 1.293 | 0.771 | 0.717 | 1.629 |
| ČܽāŠŌ | - | ¼«Ņ×ČÜ | ¼«ÄŃČÜ | ¼«Ņ×ČÜ |
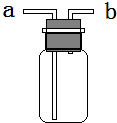
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗæĪĢĆČż¼¶½²Į·»Æѧ””””¾ÅÄź¼¶(ÉĻ) ĢāŠĶ£ŗ058
±ķÖŠĮŠ³öĮĖæÕĘų”¢NH3(°±)”¢CH4(¼×Ķé)”¢HClĖÄÖÖĘųĢåŌŚ±ź×¼×“æöĻĀµÄĆܶČŗĶČܽāŠŌ£¬¾Ż“Ė»Ų“šĻĀĮŠĪŹĢā£ŗ

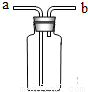
(1)ĘäÖŠ¼ČæÉÓĆÅÅĖ®·ØŹÕ¼Æ£¬ÓÖæÉÓĆĻņĻĀÅÅæÕĘų·ØŹÕ¼ÆµÄĘųĢåŹĒ________£»
(2)ČōÓĆĶ¼×°ÖĆŹÕ¼ÆNH3£¬ŌņĘųĢåÓ¦“Ó________¶ĖÖĆČė(Ģīa»ņb)£»
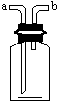
(3)ČōÓĆĶ¼×°ÖĆ³żČ„H2ÖŠµÄĖ®ÕōĘų£¬Ōņ×°ÖĆČӦװµÄŹŌ¼ĮĪŖ________£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
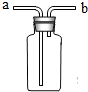 ±ķÖŠĮŠ³öĮĖæÕĘų”¢NH3£Ø°±Ęų£©”¢CH4”¢HClĖÄÖÖĘųĢåŌŚ±ź×¼×“æöĻĀµÄĆܶČŗĶČܽāŠŌ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
±ķÖŠĮŠ³öĮĖæÕĘų”¢NH3£Ø°±Ęų£©”¢CH4”¢HClĖÄÖÖĘųĢåŌŚ±ź×¼×“æöĻĀµÄĆܶČŗĶČܽāŠŌ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ| æÕĘų | NH3 | CH4 | HCl | |
| ĆÜ¶Č£Øg/L£© | 1.293 | 0.771 | 0.717 | 1.629 |
| ČܽāŠŌ | - | ¼«Ņ×ČÜ | ¼«ÄŃČÜ | ¼«Ņ×ČÜ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com