
 ����ά���������������������Ԫ�أ���ͼ��ʾΪij�ֲ��Ƽ����ƶ��桱˵�����һ���֣�ȡ1Ƭ�ƶ��棬����ʢ��10gϡ������ձ��У����е�̼��Ƹ�ϡ����ǡ����ȫ��Ӧ�������ɷ���ϡ�����Ӧ�����ձ������ʵ�����Ϊ11.34g��������㣺
����ά���������������������Ԫ�أ���ͼ��ʾΪij�ֲ��Ƽ����ƶ��桱˵�����һ���֣�ȡ1Ƭ�ƶ��棬����ʢ��10gϡ������ձ��У����е�̼��Ƹ�ϡ����ǡ����ȫ��Ӧ�������ɷ���ϡ�����Ӧ�����ձ������ʵ�����Ϊ11.34g��������㣺| �������� |
| ��Һ���� |
| 100 |
| x |
| 73 |
| y |
| 44 |
| 0.66g |
| 40 |
| 100 |
| 1.095g |
| 10g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ά���������������������Ԫ�أ���ͼ��ʾΪij�ֲ��Ƽ����ƶ��桱˵�����һ���֣�ȡ1Ƭ�ƶ��棬����ʢ��10gϡ������ձ��У�����̼��Ƹ�����ǡ����ȫ��Ӧ�������ɷ��������Ӧ�����ձ�������������Ϊ11.34g���Լ��㣺
����ά���������������������Ԫ�أ���ͼ��ʾΪij�ֲ��Ƽ����ƶ��桱˵�����һ���֣�ȡ1Ƭ�ƶ��棬����ʢ��10gϡ������ձ��У�����̼��Ƹ�����ǡ����ȫ��Ӧ�������ɷ��������Ӧ�����ձ�������������Ϊ11.34g���Լ��㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ά���������������������Ԫ�أ���ͼ��ʾΪij�ֲ��Ƽ����ƶ��桱˵�����һ���֣��Խ����йصļ��㣺
����ά���������������������Ԫ�أ���ͼ��ʾΪij�ֲ��Ƽ����ƶ��桱˵�����һ���֣��Խ����йصļ��㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
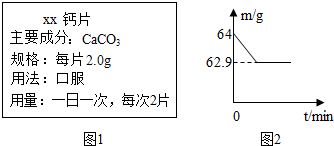 ��2009?����������ά���������������������Ԫ�أ�С�����õ�ij�ָ�Ƭ�IJ���˵����ͼ1��������֪��ÿ����õĸ�Ƭ��̼��Ƶ������������ڼ��н�����̽����ȡ2Ƭ��Ƭ�����˲������У������м���60g �״ף�����ǡ����ȫ��Ӧ�������Ƭ�������ɷֲ�����ᷴӦ������ò����������ʵ�������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ2��ʾ����Ӧ�Ļ�ѧ����ʽ��
��2009?����������ά���������������������Ԫ�أ�С�����õ�ij�ָ�Ƭ�IJ���˵����ͼ1��������֪��ÿ����õĸ�Ƭ��̼��Ƶ������������ڼ��н�����̽����ȡ2Ƭ��Ƭ�����˲������У������м���60g �״ף�����ǡ����ȫ��Ӧ�������Ƭ�������ɷֲ�����ᷴӦ������ò����������ʵ�������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ2��ʾ����Ӧ�Ļ�ѧ����ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ά���������������������Ԫ�أ�ͼΪij�ֲ��Ƽ����ƶ��桱˵�����һ���֣�ȡ1Ƭ�ƶ��棬����ʢ��10gϡ������ձ��У�����̼��Ƹ�����ǡ����ȫ��Ӧ�������ɷ��������Ӧ�����ձ�������������Ϊ11.34g������Ӧ����ʽ��CaCO3+2HCl�TCaCl2+H2O+CO2����
����ά���������������������Ԫ�أ�ͼΪij�ֲ��Ƽ����ƶ��桱˵�����һ���֣�ȡ1Ƭ�ƶ��棬����ʢ��10gϡ������ձ��У�����̼��Ƹ�����ǡ����ȫ��Ӧ�������ɷ��������Ӧ�����ձ�������������Ϊ11.34g������Ӧ����ʽ��CaCO3+2HCl�TCaCl2+H2O+CO2�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com