
·ÖĪö £Ø1£©øł¾Ż¹żĀĖŹ±ĖłÓĆŅĒĘ÷¼°²£Į§°ōµÄ×÷ÓĆ½ā“š£»
£Ø2£©øł¾Ż·“Ó¦Į÷³Ģ¼°·“Ó¦ĪļŗĶÉś³ÉĪļŠ“³ö»Æѧ·½³ĢŹ½£»
£Ø3£©øł¾Ż·“Ó¦Į÷³Ģ·ÖĪö½ā“š£»
£Ø4£©øł¾Ż·“Ó¦ŗóČÜŅŗµÄĪŪČ¾ŠŌ·ÖĪö£»
£Ø5£©ŅŖµĆµ½øü¶ąµÄĶ£¬æɽ«·“Ó¦¹ż³ĢÖŠµÄĶČ«²æ»ŲŹÕ£®
½ā“š ½ā£ŗ
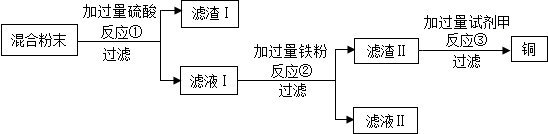
£Ø1£©øł¾Ż¹żĀĖŹ±ĖłÓĆŅĒĘ÷æÉÖŖ£¬±ŲŠėÓƵ½µÄ²£Į§ŅĒĘ÷ÓŠÉÕ±”¢²£Į§°ōŗĶĀ©¶·£¬ĘäÖŠ²£Į§°ōµÄ×÷ÓĆŹĒŅżĮ÷
£Ø2£©Ńõ»ÆĶÓėĮņĖį·“Ӧɜ³ÉĮņĖįĶŗĶĖ®£¬Ķ”¢Ä¾ĢæÓėĮņĖį²»·“Ó¦£¬»Æѧ·½³ĢŹ½ĪŖ£ŗH2SO4+CuOØTCuSO4+H2O£»
£Ø3£©·“Ó¦¢Łŗó£¬ĀĖŅŗ1ÖŠµÄČÜÖŹŹĒĮņĖįŗĶĮņĖįĶ£¬¹żĀĖŗóµÄĀĖŅŗÖŠ¼ÓČė¹żĮæĢś·Ū£¬ĮņĖįĶŗĶĢś·Ū·“Ӧɜ³ÉĮņĖįŃĒĢśŗĶĶ£¬»Æѧ·½³ĢŹ½ĪŖ£ŗFe+CuSO4ØTFeSO4+Cu£¬H2SO4+FeØTFeSO4+H2”üŹōÓŚÖĆ»»·“Ó¦£»ĀĖŅŗ¢ņÖŠµÄČÜÖŹĪŖĮņĖįŃĒĢś£»
£Ø4£©ĀĖŌü¢ņÖŠŗ¬ÓŠŹ£ÓąµÄĢś·Ū£¬¾¹ż¢ŪŗóµĆµ½Ķ£¬æÉÖŖ³żČ„¹żĮæµÄĢś·Ū£¬×īŗĆÓĆĮņĖįČÜŅŗ£¬ÓĆĮņĖįĶ·“Ó¦Ņ»¶ĪŹ±¼äŗó£¬Ģś±ķĆęÓŠĶ»į×č°ĢśµÄČܽā£»
£Ø5£©¹żĮæµÄĢśŗĶĮņĖį·“Ӧɜ³ÉĒāĘų£¬»į擵½ÓŠĘųÅŻĆ°³ö£¬µ±²»ŌŁÓŠĘųÅŻĆ°³ö£¬ĮņĖį¹żĮ森
¹Ź“š°øĪŖ£ŗ
£Ø1£©Ā©¶·£»ŅżĮ÷£»
£Ø2£©H2SO4+CuOØTCuSO4+H2O£»
£Ø3£©ÖĆ»»·“Ó¦£»FeSO4£»
£Ø4£©C£»
£Ø5£©²»ŌŁÓŠĘųÅŻĆ°³ö£®
µćĘĄ ¶ŌÓŚĮ÷³ĢĶ¼µÄĢāÄæ¹Ų½”ŹĒæ“Ć÷°×Į÷³ĢĶ¼ÖŠø÷ĪļÖŹµÄ×Ŗ»Æ¹ŲĻµ£¬×¢Ņā¹żĮæĪļÖŹµÄČ„Ļņ£¬ČēŗĪ³żČ„¹żĮæĪļÖŹ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Éś³ÉĘųĢåµÄÖŹĮææÉÄÜŹĒ11æĖ»ņ33æĖ | |
| B£® | ĀĖŌüÖŠµÄŗģÉ«ĪļÖŹæÉÄÜŹĒ32æĖ»ņ96æĖ | |
| C£® | ²ĪÓė·“Ó¦µÄŃõ»ÆĶÖŹĮææÉÄÜŹĒ40æĖ»ņ120æĖ | |
| D£® | Ļ”ĮņĖįµÄČÜÖŹÖŹĮææÉÄÜŹĒ38.4æĖ»ņ115.2æĖ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

| ·“Ó¦Ē° | ·“Ó¦ŗó |
| EµÄÖŹĮæĪŖ100.0g | EµÄÖŹĮæĪŖ102.25g |
| FµÄÖŹĮæĪŖ50.0g | FµÄÖŹĮæĪŖ51.1g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
| ĪĀ¶Č£Ø”ę£© | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
| Čܽā¶Č£Øg/100gĖ®£© | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 | 138 | 169 | 202 | 246 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com