��2013?�����ģ�⣩��ѧ������������أ�
��1���ճ�������ʳ�õļ�������֬���и�����Ӫ������
������
������
��Ϊ�˷�ֹ���Ͳ�����������������������Բ���һЩ��
��
��
Ԫ�ص�ʳƷ��
��2��������Ʒ��ʹ�õ���Ҫ����������Ȼ��ά����
C
C
������ţ���ͬ����

��3�������˵�θҺ�к���Լ0.5%�����ᣮij��θҩ�к���Al��OH��
3��������θ�����ļ�������ҩ�����Ļ�ѧ��Ӧ����ʽΪ
Al��OH��3+3HCl�TAlCl3+3H2O
Al��OH��3+3HCl�TAlCl3+3H2O
���÷�Ӧ����
���ֽⷴӦ
���ֽⷴӦ
����д������Ӧ���ͣ���
��4����֪Ba
2+�о綾������̼�ᱵ�����ᱵ�����Σ�������ҽ���ϲ�������X������θ�����ڷ�ҩ����
BaCO3
BaCO3
���û�ѧʽ��ʾ����
��5��A��B���������dz��нγ������������ͬԪ����ɵģ�
����A��B��Ϊ���壬��A��B���Ի���ת������A��B�Ļ�ѧ����ʽΪ
��
����A��B����Һ�����ת��Ϊ����C����C�Ļ�ѧʽΪ
O2
O2
��
����A������������Ҫ���ʣ���ҵ�������������ͨ�����з�Ӧ�õ�A��4FeS
2+11O
2�T2Fe
2O
3+8A
�ڿ����У�A��������ϵ��ת���������γ����꣺A
B
���ᣬ��B���ʵĻ�ѧʽΪ
SO3
SO3
��




 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�

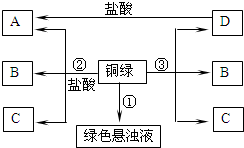 ��2013?�����ģ�⣩Сͮ��һ���۾�����һ��ʱ�����ͭ�ʾ����ϳ�����һ����ɫ���ʣ����뽫����������������ϵ�֪��ͭ��һ�������»���ʴ����һ����ɫ���ʣ�����Ҫ�ɷ��Ǽ�ʽ̼��ͭ[�׳�ͭ�̣���ѧʽΪCu2��OH��2CO3]�����������Ȳ��ȶ��ֽ⣮������Ԫ���غ�������ó�ͭ������ͭ������е�������������̼��
��2013?�����ģ�⣩Сͮ��һ���۾�����һ��ʱ�����ͭ�ʾ����ϳ�����һ����ɫ���ʣ����뽫����������������ϵ�֪��ͭ��һ�������»���ʴ����һ����ɫ���ʣ�����Ҫ�ɷ��Ǽ�ʽ̼��ͭ[�׳�ͭ�̣���ѧʽΪCu2��OH��2CO3]�����������Ȳ��ȶ��ֽ⣮������Ԫ���غ�������ó�ͭ������ͭ������е�������������̼��