
 Č¼ÉÕ”¢Ćš»šÓėĪŅĆĒµÄÉś»īŗĶÉś²śÓŠ×ÅĆÜĒŠµÄĮŖĻµ£®ĻĀĶ¼ŹĒČ¼ÉÕĢõ¼žŹ¾ŅāĶ¼£ŗ
Č¼ÉÕ”¢Ćš»šÓėĪŅĆĒµÄÉś»īŗĶÉś²śÓŠ×ÅĆÜĒŠµÄĮŖĻµ£®ĻĀĶ¼ŹĒČ¼ÉÕĢõ¼žŹ¾ŅāĶ¼£ŗ
 2CO2+3H2O
2CO2+3H2O 2CO2+2CO+6H2O
2CO2+2CO+6H2O 2CO2+2CO+6H2OÖŠ£¬µČŗÅĮ½±ßŌ×ÓµÄÖÖĄąŗĶŹżÄæĻąµČ£¬ĖłŅŌ·“Ó¦ŅŅÖŠµÄ»Æѧ¼ĘĮæŹżaµČÓŚ2£¬£»
2CO2+2CO+6H2OÖŠ£¬µČŗÅĮ½±ßŌ×ÓµÄÖÖĄąŗĶŹżÄæĻąµČ£¬ĖłŅŌ·“Ó¦ŅŅÖŠµÄ»Æѧ¼ĘĮæŹżaµČÓŚ2£¬£» 2CO2+2CO+6H2O
2CO2+2CO+6H2O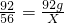


 ½Ģ²ÄČ«½ā×Ö“Ź¾äĘŖĻµĮŠ“š°ø
½Ģ²ÄČ«½ā×Ö“Ź¾äĘŖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø1£©2011Äźæµ·ĘĀ©ÓĶŹĀ¼žÖŠ£¬Š¹Ā©µÄČ¼ÓĶ¶Ōŗ£Ńó»·¾³ŗĶÖܱßŅ°ÉśÉśĪļŌģ³ÉµÄ¾Ž“óµÄÉśĢ¬ŌÖÄŃ”£ŹÆÓĶÓėĢģČ»ĘųĻą°é¶ųÉś”£ŹŌŠ“³öĢģČ»ĘųČ¼ÉյĻÆѧ·½³ĢŹ½”£
£Ø2£©2012Äź1ŌĀ13ČÕ£¬ŗÓÄĻŹ”²©°®ŠĀŹĄ¼ĶÉś»ī¹ć³”·¢Éś»šŌÖ£¬ŹĀ¹Ź·¢ÉśŹ±ÉĢĻĆ»¹ŌŚÓŖŅµ”£Ļū·Ą¶ÓŌ±ŃøĖŁÓĆ×ŌĄ“Ė®Ćš»š”£¢ŁŹŌ·ÖĪöĖūĆĒĆš»š·½·ØĖłŅĄ¾ŻµÄŌĄķŹĒŹ²Ć“£æ¢ŚČē¹ūĪŅĆĒŹĒŅ»ĆūĻÖ³”Ź©¾ČµÄ![]() Ö¾ŌøÕߣ¬Ó¦ČēŗĪ×öŗĆ×ŌĪŅ·Ą»¤“ėŹ©£¬²¢ĖµĆ÷ŌŅņ”£
Ö¾ŌøÕߣ¬Ó¦ČēŗĪ×öŗĆ×ŌĪŅ·Ą»¤“ėŹ©£¬²¢ĖµĆ÷ŌŅņ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012½ģŗÓÄĻŹ”ÖŠÕŠæ¼ŹŌĖµĆ÷½āĆÜŌ¤²ā£ØĖÄ£©»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĪŹ“šĢā
£Ø1£©2011Äźæµ·ĘĀ©ÓĶŹĀ¼žÖŠ£¬Š¹Ā©µÄČ¼ÓĶ¶Ōŗ£Ńó»·¾³ŗĶÖܱßŅ°ÉśÉśĪļŌģ³ÉµÄ¾Ž“óµÄÉśĢ¬ŌÖÄŃ”£ŹÆÓĶÓėĢģČ»ĘųĻą°é¶ųÉś”£ŹŌŠ“³öĢģČ»ĘųČ¼ÉյĻÆѧ·½³ĢŹ½”£
£Ø2£©2012Äź1ŌĀ13ČÕ£¬ŗÓÄĻŹ”²©°®ŠĀŹĄ¼ĶÉś»ī¹ć³”·¢Éś»šŌÖ£¬ŹĀ¹Ź·¢ÉśŹ±ÉĢĻĆ»¹ŌŚÓŖŅµ”£Ļū·Ą¶ÓŌ±ŃøĖŁÓĆ×ŌĄ“Ė®Ćš»š”£¢ŁŹŌ·ÖĪöĖūĆĒĆš»š·½·ØĖłŅĄ¾ŻµÄŌĄķŹĒŹ²Ć“£æ¢ŚČē¹ūĪŅĆĒŹĒŅ»ĆūĻÖ³”Ź©¾ČµÄÖ¾ŌøÕߣ¬Ó¦ČēŗĪ×öŗĆ×ŌĪŅ·Ą»¤“ėŹ©£¬²¢ĖµĆ÷ŌŅņ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012ÄźŗÓÄĻŹ”ČżĆÅĻæŹŠŹµŃéÖŠŃ§ÖŠæ¼»ÆѧŅ»Ä£ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com