�����д����л�ѧ����ʶ��̽�����ߵĻ�ѧ���ʣ���ѧϰ��ѧ����Ҫ���ݣ�������ѧ�Ļ�ѧ֪ʶ��գ�
��1�������dz����г��õĴ��ߣ�����˵��һ����ֹ��������ķ�����
���ֽྻ����
���ֽྻ����
��
��2��С���������ϸ���ʱ������һЩʳ�ף������ϸ�������ˣ��������Dz����ɺ��أ�������ȡ�ϸ�����֭Һ�ظ���ʳ�ף�������ͬ���������ϸ���֭Һ�м���һЩ�����������ϸ���֭Һ���̣������С��һ�����������⣺
��ʳ��pH
��
��
7���������=��������������������
��
��
����ᡱ��������Ρ�������
�ڽ��С����ʵ�飬���������ϸ���֭Һ����;
�����ָʾ��
�����ָʾ��
��
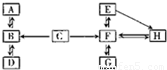
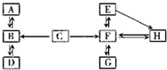
��3��A-H�dz��л�ѧ�еij������ʣ���������֮���ת����ϵͼ���ش��������⣮��ͼ�С�������ʾ���ʼ����ת����ϵ��
��A�ڱ�״�������ܶ���С�����壬A�Ļ�ѧʽ��
H2
H2
��A��B��C�к���ͬһ��Ԫ�أ�C��Bʱ��Һ�ʻ�ɫ���ɴ��ƶ���C��Ӧ����B��������
Fe2O3
Fe2O3
��
��DΪ���嵥�ʣ�д��B��D�Ļ�ѧ����ʽ
��

 �����д����л�ѧ����ʶ��̽�����ߵĻ�ѧ���ʣ���ѧϰ��ѧ����Ҫ���ݣ�������ѧ�Ļ�ѧ֪ʶ��գ�
�����д����л�ѧ����ʶ��̽�����ߵĻ�ѧ���ʣ���ѧϰ��ѧ����Ҫ���ݣ�������ѧ�Ļ�ѧ֪ʶ��գ�

 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�


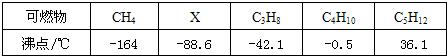
 �����д����л�ѧ����ʶ��̽�����ߵĻ�ѧ���ʣ���ѧϰ��ѧ����Ҫ���ݣ�������ѧ�Ļ�ѧ֪ʶ��գ�
�����д����л�ѧ����ʶ��̽�����ߵĻ�ѧ���ʣ���ѧϰ��ѧ����Ҫ���ݣ�������ѧ�Ļ�ѧ֪ʶ��գ�