
��8�֣���ѧ�����������ߣ��������ǵ�����ϢϢ��ء�
�� ����H��C��O��Ca���ֳ�����Ԫ�أ���ѡ�����е�Ԫ��д����������Ҫ������ʸ�һ�֣��û�ѧʽ��ʾ����
��������ܼ�________________ �ڴ���ʯ����Ҫ�ɷ�___________
�ۿ����ڸ������������ļ���____________�ܿ���������ζƷ����___________
�� ��ʵġ�̼�������˵����������һ���߽���̼�������硣
�ٻ���̿�ɾ�ˮ��˵������̿����___________�ԣ�
��Һ̬������̼������������˾ȵ��������ҵĻ��֣�����˵����ȷ����_______________��
A��Һ̬������̼��������Ⱦ��������
B��������̼�ɸ����ڿ�ȼ����棬�����˿���
C��Һ̬������̼����ʱ���ȣ������˿�ȼ����Ż��
�۹���Ķ�����̼�Ӿ�������ЧӦ��д��һ�����ٶ�����̼�ŷŵĽ���_________��
��Ŀǰ�������Ի�ʯȼ��Ϊ��Ҫ��Դ����ʯȼ����ú��ʯ�ͺ�__________��
�Ţ�H2O ��CaCO3�� Ca(OH)2��CH3COOH��C12H22O11��C2H5OH �Ƣ����� �� A��B �ۼ��ٻ�ʯȼ�ϵ�ȼ�գ������𰸾����֣�����Ȼ��
�������������������֪Ԫ�ص���ϼ��������ʵ����ʿ�֪��1����������ܼ���H2O �ڴ���ʯ����Ҫ�ɷ���CaCO3�ۿ����ڸ������������ļ����������ƻ�ѧʽΪCa(OH)2�ܿ���������ζƷ����Ϊʳ����ΪCH3COOH����2���ٻ���̿�ɾ�ˮ��˵������̿���������ԣ���Һ̬������̼������������˾ȵ��������ҵĻ�������ΪҺ̬������̼��������Ⱦ�������ϣ�������̼�ɸ����ڿ�ȼ����棬�����˿������۹���Ķ�����̼�Ӿ�������ЧӦ��Ϊ���ٶ�����̼�ŷ�Ӧ�ü��ٻ�ʯȼ�ϵ�ʹ�ã���Ŀǰ�������Ի�ʯȼ��Ϊ��Ҫ��Դ����ʯȼ����ú��ʯ�ͺ���Ȼ����
���㣺��ѧ�����̼��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣�ˮ�DZ������Ȼ��Դ����һ����������������ģ�����Ӧ���˽��й�ˮ��һЩ֪ʶ����ش��������⣺
��1������ˮ������ˮ����Ȫˮ����ˮ�����ڴ�������� ��
��2���þ�ˮϴ�·�ʱ���������������Ҳ�����������ԭ���Ǿ�ˮ������ ��ѡ�Ӳˮ������ˮ������
��3����ͼ��һ�ּ��û���������Һ�����������з�������Ҫ��Ӧ���Ȼ��ƺ�ˮ��ͨ�������·�Ӧ�����������ơ�������������Cl2�����÷�Ӧ�Ļ�ѧ����ʽΪ ���������仯�Ƕȿ����÷�Ӧ�� ��ת��Ϊ��ѧ�ܡ�
��4������1000g������������15%��ʳ��ˮ����Ҫʳ�� g��Ҳ���������ʷ���25%��ʳ��ˮ g��ˮϡ�Ͷ��ɡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣�Ϊ�������������ŷţ����ǻ���Ѱ�Ҳ���̼Ԫ�ص�ȼ�ϡ����о�����NH3ȼ�յIJ���û����Ⱦ�����ͷŴ�����������һ��Ӧ��ǰ��������ʾ��ͼ������ʾ��
��1��������ѧ��Ӧ�����У������������� _________������ţ���
��2��A�����е�Ԫ�صĻ��ϼ�Ϊ_________�ۣ��ڻ�ѧ��Ӧǰ��Ԫ�ػ��ϼ�û�з����ı����__________Ԫ�أ�
��3���÷�Ӧ�Ļ�ѧ����ʽΪ______________���������Ӧ��������___________��
��4�� �μӷ�Ӧ��A��B���������������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(7��)��ͼ���й�������֪ʶ����ͼ(��Ӧ����������ȥ)���û�ѧ������գ�
��1�� ���ʢٵĻ�ѧʽ ��
��2�� ���ʢ��е������� ��
��3�� ���ʢ۵Ļ�ѧʽ ��
��4�� ���ʢ�����Ԫ�ص����� ��
��5�� ������ʢݻ�ѧʽ�д���Ԫ�صĻ��ϼ� ��
��6�� �������ʢĻ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ͼ��Ӧģ��ͼ�ش�
��1�����÷��ű�ʾAʾ�������� ��
��2����д���˷�Ӧ�ķ���ʽ ��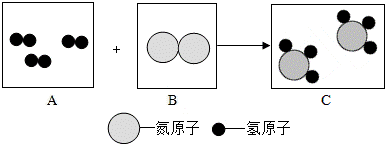
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��4 �֣����� 60 ���������ҹ���ѧ������ѧ�ķ�չ��ȡ���˾�����Ŀ�ijɾ͡��ҹ���ѧ������ֽ�Ͷ������ѣ�TiO2����Ĥ������һ�����͡�����ֽ������������ֽ�ϡ��̡�һ�㡰��������C10H9N��Ⱦ�ϣ��Ƴ�һ����ֽ�����ڼ��ʳƷ����������Ũ�ȵĸߵ͡�
��1������������ Ti �Ļ��ϼ�Ϊ ��
��2��������һ�������У�C��H��N ����ԭ�ӵĸ���֮��Ϊ ������������ȣ���
��3����֪ NaNO2 �ܷ������·�Ӧ����ѧ����ʽ����ƽ����
2NaNO2+2KI+2H2SO4=I2+2M��+2H2O+Na2SO4+K2SO4���� M �Ļ�ѧʽ�� ��
��4������������Ӧ�����û�ѧ��ֽ�������г������ʽ���ʵ���������������κ�ʳ�Σ����е⻯�ص�����ֽ������ѡ�����������еij�������Ϊ________������ţ���
��ʳ���� �ڰ� ��ʳ�� ��ţ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��3�֣�ij��һ�Ҵ��ͻ������������պ�ͭ���ϵ������������£�
���̣�1���õ��IJ����ﲻ����ˮ��ϡ������̣�2���Ǻ�ɫ������ϡ���������������ͭ��ˮ����д������������������ֱͭ���йصĻ�ѧ����ʽ��
��1��
��2��
��3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
д����������Ҫ��Ļ�ѧ���
���Ȼ����е������� �� ��������ֽ��ϴ�Ӽ��ļ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijԪ��Rԭ�ӵĺ˵����Ϊ1��18֮�䣬��֪R2-�ĺ�����x�����ӣ���Rԭ�ӵĺ����������������ƻ���ʱ�Ļ�ѧʽ�ֱ��� �� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com