�⣺��1������������Һ���ʵ�����Ϊ��10%��500g=50g�������ܼ�ˮ������Ϊ��500g-50g=450g��
����ˮ���ܶ�Ϊ1g/ml������ˮ�����Ϊ450ml���ʴ�Ϊ��450
��2��Aѡ�������ʹ�����������Ƹ�����ֽ�ϣ�ʹ���ʼ��٣������������ƫС��
Bѡ����ˮʱ�����ӿ̶ȣ������ˮƫ�٣���ʹ��������ƫ��
Cѡ���ʹ�ܼ����٣������������ƫ��
�ʴ�Ϊ��A
��3����������Һ��켴��֤����̪û�б��ʣ���ʵ�������ϣ����Բ��벻��ȷ��
��ͨ��ʵ������ɿ������ڶ�����������Һ������ϡ�ͣ���Һ����ɫ�����˸ı䣬������Һ��Ũ���뷴Ӧ�йأ�
�ʴ�Ϊ��������̪���ʣ�������ֺ�ɫ��
���������Ƶ�Ũ��
��4������������һ�ּ���ԺͿ����ж�����̼��Ӧ����̼���ƺ�ˮ��2NaOH+CO
2�TNa
2CO
3+H
2O�����Ա���ʱ�����ܷ⣮
�ʴ�Ϊ��2NaOH+CO
2�TNa
2CO
3+H
2O���ܷ�
��5����Ϊ�������Ʊ���������̼�������ɣ����Կɼ���һ�����ʺ�̼���Ʒ�Ӧ��������������û�����ɣ�
��μӸ����ӵ���Һ���߱����ӵ���Һ��������ְ�ɫ������
�ʴ�Ϊ����������������ˮ���μ�����������Һ�����Ȼ��ơ��Ȼ���������������Һ�ȣ����а�ɫ��������
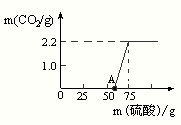
��6���⣺��ͼ����֪��Ӧ��ʼ����50ml�����Ǻ��������Ʒ�Ӧ����������20ml�����Ǻ�̼���Ʒ�Ӧ���������˶�����̼����2.2g
�����ɵ�̼��������Ϊx
Na
2CO
3+H
2SO
4�TNa
2SO
4+H
2O+CO
2��
106 44
x 2.2g
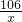
=
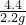
��֮�ã�x=5.3g
�����������Ƶ�����Ϊ��10.6g-5.3g=5.3g
�������Ƶ���������Ϊ��
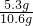
��100%=50%
����Ʒ��NaOH����������Ϊ50%
��������1���ɸ������ʵ����������Ķ����ʽ������⣮
��2�����Ƶ���Һ��������ƫС���������������Ķ����֪��������������������������ٻ��ܼ�ˮ����ƫ��
��3����Ϸ�̪��������Һ������ʵ������������ʵ�ۺϷ������
��4������������һ�ּ���ԺͿ����ж�����̼��Ӧ�����κ�ˮ��
��5���������Ʊ�������̼���ƣ��ɸ���̼���Ƶ����ʺͷ�Ӧ���������ƣ�
��6�����ȷ���ͼ�����ݶ�Ӧ�ķ�Ӧ��0-50ml�����Ǻ��������Ʒ�Ӧ��50-75ml�����Ǻ�̼���Ʒ�Ӧ��������2.2g���壮
����������������ʵ��̽���⣬����ʵ����̵�̽�������н��۵�̽�������й�����Һ��ϡ��������йؼ��㣬�ۺ��ԱȽ�ǿ������������⡢�������裬Ȼ�����ʵ�鷽��������ʵ�飬���ó���ȷ�Ľ��ۣ�������Ƶķ�����������չ�����������п�����Ҫ����֮һ����Ҫ������ʵ�����У�

 ������ij�о���ѧϰС������������й����ʽ���̽���Ĺ��̣���ش��������⣮
������ij�о���ѧϰС������������й����ʽ���̽���Ĺ��̣���ش��������⣮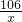 =
=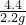
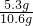 ��100%=50%
��100%=50%

 ��У����ϵ�д�
��У����ϵ�д�

 ������ij�о���ѧϰС������������й����ʽ���̽���Ĺ��̣���ش��������⣮
������ij�о���ѧϰС������������й����ʽ���̽���Ĺ��̣���ش��������⣮