
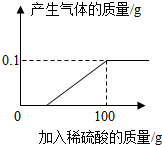
 ����пƬ��ҽҩ�������������ʳ��������������֢״��ʵ���ҿ���п��ϡ���ᷴӦ�Ƶã�ѧУ��ѧ��ȤС���ͬѧȡһ���������������пƬ7.3g�������������ʣ������飬�����������μ�9.8%��ϡ���ᣬ��ǡ����ȫ��Ӧʱ����ȥϡ����100g���������ͼ��ʾ��ͼ��С��ͬѧ����Ӧ�����Һ��һϵ�в������õ�����п16.1g��
����пƬ��ҽҩ�������������ʳ��������������֢״��ʵ���ҿ���п��ϡ���ᷴӦ�Ƶã�ѧУ��ѧ��ȤС���ͬѧȡһ���������������пƬ7.3g�������������ʣ������飬�����������μ�9.8%��ϡ���ᣬ��ǡ����ȫ��Ӧʱ����ȥϡ����100g���������ͼ��ʾ��ͼ��С��ͬѧ����Ӧ�����Һ��һϵ�в������õ�����п16.1g������ ��1���ٸ�����Һϡ��ǰ������������Ƚ��з�����
�ڸ����Լ�ƿ����Ҫע��ҩƷ�����ƺ����������������з�����
�۸��ݵ������Ƶ���Һ�����ʵ�������������9.8%�Ŀ���ԭ�����������˻��ܼ����˽��з�����
��2������п�����ᷴӦ��������п�������������������������м��㣻
��3���������ĵ���������е�ͼ����м��㣮
��� �⣺��1������98%��Ũ��������200g9.8%��ϡ���ᣬ���ˮ������Ϊ��200g-$\frac{200g��9.8%}{98%}$=180g��
���Լ�ƿ����Ҫע��ҩƷ�����ƺ�����������������ѡ��BC��
�۵������Ƶ���Һ�����ʵ�������������9.8%�Ŀ���ԭ�����������˻��ܼ����ˣ�
A����ȡŨ��������ڿ�����ʱ�����������Ũ��������������ˣ�����������������ƫ�ͣ���A��ȷ��
B����ˮʱ��������Ͳ�̶ȣ�ˮ��������ˣ�����������������ƫ��B����
C������ǰ���ձ���ˮ��ϴ�ɾ���û�в��ɣ������ܼ����������ˣ�����������������ƫ�ͣ���C��ȷ��
��ѡ��AC��
��2��������0.1g�������ĵ�ϡ�����������x
Zn+H2SO4=ZnSO4+H2��
98 2
x��9.8% 0.1g
$\frac{98}{x��9.8%}$=$\frac{2}{0.1g}$
x=50g
��3��п�����ᷴӦ��������п������������п�����ᷴӦ��������п��ˮ��������������������غ��֪
���пƬû������ǰ��������y
Zn--------H2SO4��
65 98
y 100g��9.8%
$\frac{65}{y}$=$\frac{98}{100g��9.8%}$
y=6.5g
�ʴ�Ϊ����1����180g��
��BC��
��AC��
��2��50g��
��3��6.5��
���� ������Ҫ�����˻�ѧ����ʽ�ļ��㣬�ѶȲ���ע�����Ĺ淶�Ժ�ȷ�ԣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
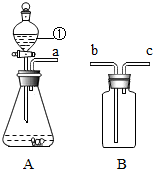 ��ͼA��Bװ����Ͽ�����O2��H2��CO2�����ʵ�����Ʊ��������ٵ������Ƿ�Һ©������ѡ��Aװ����ȡO2����ѧ����ʽΪ2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2��������Bװ���ռ�CO2���壬��a��b���b����c����������Bװ��ˮ�������ռ�A�����ɵ�H2����a��c���b����c������
��ͼA��Bװ����Ͽ�����O2��H2��CO2�����ʵ�����Ʊ��������ٵ������Ƿ�Һ©������ѡ��Aװ����ȡO2����ѧ����ʽΪ2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2��������Bװ���ռ�CO2���壬��a��b���b����c����������Bװ��ˮ�������ռ�A�����ɵ�H2����a��c���b����c�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2O��H2O2�����Ԫ����ͬ���������ǵĻ�ѧ������ͬ | |
| B�� | ��ij�����еμ�ϡ���ᣬ�����������ݣ��������в�һ������CO32- | |
| C�� | ���ӡ�ԭ�Ӷ����Թ������ʣ��������ʶ����ɷ��ӡ�ԭ�ӹ��ɵ�? | |
| D�� | ��ͬһ�������У�����Ԫ�������ۣ����Էǽ���Ԫ��һ���Ը��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��H2O��H2O2�����Ԫ����ͬ�������ǵĻ�ѧ���ʲ�ͬ | |
| B�� | �����ơ�п��������������Ԫ�أ��������ܹ������� | |
| C�� | ����ͨ����ˮ�ķ�����������李�ʳ�κ����������ְ�ɫ��ĩ | |
| D�� | ��̼ȼ��ȼ�ղ����ʱ�ײ����綾��CO������ȼ��ʱ���뱣֤ͨ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��Ȼ�����ӱ�����ˮ�����У��ڵ������ѹ�½ᾧ�γ�������һ�������ʣ���������ȼ�գ���ͼ�������Ա���������ȼ�����������йء���ȼ������˵����ȷ���ǣ�������
��Ȼ�����ӱ�����ˮ�����У��ڵ������ѹ�½ᾧ�γ�������һ�������ʣ���������ȼ�գ���ͼ�������Ա���������ȼ�����������йء���ȼ������˵����ȷ���ǣ�������| A�� | ���IJ��ַ����Ѳ����˶� | B�� | ���Ļ�ѧ������ˮ���� | ||
| C�� | ���ķ���֮��û�м�� | D�� | ����ȫȼ�ղ�����CO2��H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����̼����--ϡ�����Ca��OH��2��Һ | |
| B�� | �������--�ӵ�ʳ�� | |
| C�� | ����NaCl��NaOH��Һ--��ɫʯ����Һ | |
| D�� | ������ˮ��Ӳˮ--����ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com