
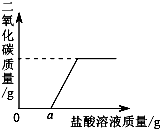
 һƿ���õ��������ƹ����Ѿ������˱��ʣ�ij�о���ѧϰС��Ϊ��̽�����ʳ̶ȣ��������²��룺���ܲ��ֱ��ʣ�������NaOH��Na2CO3�Ļ�������ȫ�����ʣ�������Na2CO3��
һƿ���õ��������ƹ����Ѿ������˱��ʣ�ij�о���ѧϰС��Ϊ��̽�����ʳ̶ȣ��������²��룺���ܲ��ֱ��ʣ�������NaOH��Na2CO3�Ļ�������ȫ�����ʣ�������Na2CO3��| ��Ʒ���� | ��Ӧǰ������ | ��Ӧ�������� |
| 10.6g | 148.5g | 146.3g |
| 106 |
| 44 |
| x |
| 2.2g |
| 5.3g |
| 10.6g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ | B������ͭ |
| C��ϡ���� | D���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���������� |
| B��Ũ����ϡ�� |
| C��������Һ���� |
| D���������Ƴ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��п��ϡ���� |
| B���ռ���ϡ���� |
| C��������ͭ��ϡ���� |
| D������ͭ��Һ������������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
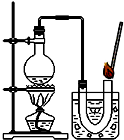 С����֤������������˫��ˮ�ķֽⷴӦ��������ã��������ڷ�Ӧǰ�������Ƿ����仯��������ͼʵ�飮
С����֤������������˫��ˮ�ķֽⷴӦ��������ã��������ڷ�Ӧǰ�������Ƿ����仯��������ͼʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
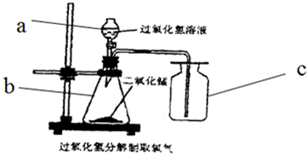 ʵ�����У������÷ֽ����������Һ����������������������ȸ�����صķ�����ȡ������
ʵ�����У������÷ֽ����������Һ����������������������ȸ�����صķ�����ȡ�������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com