


 ÖÜÖÜĒå¼ģ²āĻµĮŠ“š°ø
ÖÜÖÜĒå¼ģ²āĻµĮŠ“š°ø ĒįĒɶį¹ŚÖܲāŌĀæ¼Ö±ĶØøßæ¼ĻµĮŠ“š°ø
ĒįĒɶį¹ŚÖܲāŌĀæ¼Ö±ĶØøßæ¼ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
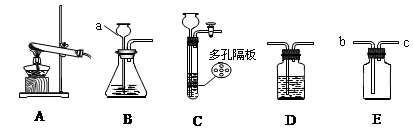
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŌŚ¹żŃõ»ÆĒāÖŠ¼ÓČė¶žŃõ»ÆĆĢ”””””””””””” | B£®¼ÓČČŃõ»Æ¹Æ”””” |
| C£®Ö²ĪļµÄ¹āŗĻ×÷ÓĆ”””””””””””””” | D£®Ö²ĪļµÄŗōĪü×÷ÓĆ |
²éæ““š°øŗĶ½āĪö>>
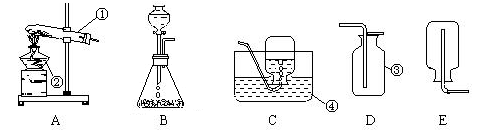
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
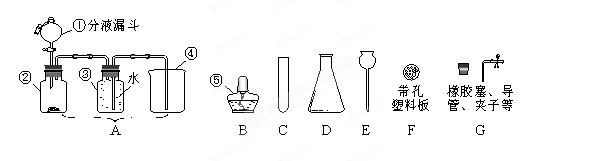
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
²éæ““š°øŗĶ½āĪö>>
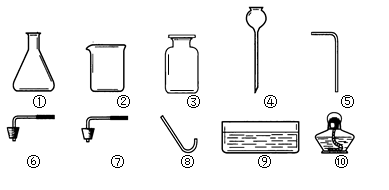
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
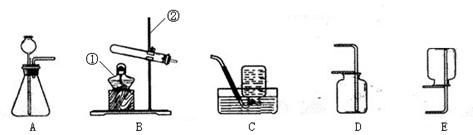
| ĘųĢå | ¼ģŃé·½·Ø | ŹµŃéĻÖĻó |
| O2 | | |
| CO2 | | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
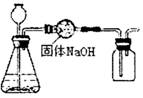
| A£®Zn£«H2SO4£½ZnSO4£«H2”ü |
B£®2H2O2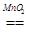 2H2O£«O2”ü 2H2O£«O2”ü |
| C£®CaCO3£«2HCl£½CaCl2£«H2O£«CO2”ü |
D£®2NH4Cl£«Ca(OH)2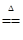 CaCl2£«2NH3”ü£«2H2O CaCl2£«2NH3”ü£«2H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com