
С�Ʒ���ůˮƿ����һ�㵭��ɫ��ˮ������Ϥ���õ�����ˮ���г���̼����⣬���������࣮����̽������û���������ʣ��������ռ�ˮ�����º�ɽ�������ʵ�飺


��1��ȡ2.5gˮ����ͼ���е�Aװ�ø��¼��ȳ�ַ�Ӧ����֪CaCO3
 CaO+CO2��������������������ͨ��B��Cװ�ã�ʵ�����Cװ��������������Һ������0.88g��
CaO+CO2��������������������ͨ��B��Cװ�ã�ʵ�����Cװ��������������Һ������0.88g��
��д��Cװ���з��ֵĻ�ѧ��Ӧ����ʽ����
��Bװ���е�Ũ�������������շ�Ӧ�в�����ˮ����������������Ũ����������ԣ�
�۸�ˮ������̼��Ƶ���������Ϊ������
��2����ͼ����ʾ�ķ�����ʵ�飬������ֳ���ʯ��ˮû�б���ǣ�ԭ������������
�����㡿ʵ��̽�����ʵ���ɳɷ��Լ�������
��ר�⡿ѹ��ʵ���⣻��ѧ̽����
������������������Һ�ܹ����ն�����̼��������������ʣ�
Ũ���������ˮ�ԡ���ˮ�ԡ�ǿ�����ԣ�
���ݻ�ѧ����ʽ���Խ�����ط���ļ��㣻
Ũ������лӷ��ԣ��ܺ��Լ��Ե����ʷ�Ӧ��
����𡿽⣺��1�����������ƺͶ�����̼��Ӧ������̼���ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��CO2 +2NaOH�TNa2CO3+H2O��
���CO2 +2NaOH�TNa2CO3+H2O��
��Ũ�����ܹ��������շ�Ӧ�в�����ˮ������˵��Ũ���������ˮ�ԣ�
�����ˮ��
��Cװ��������������Һ������0.88g��˵��̼��Ʒֽ�������0.88g������̼��
��̼��Ƶ�����ΪX��
CaCO3
 CaO+CO2��
CaO+CO2��
100 44
X 0.88g

 =
=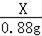

X=2g��
��ˮ������̼��Ƶ���������Ϊ��
 ��100%=80%��
��100%=80%��
���80%��
��2��Ũ������лӷ��ԣ��ӷ������Ȼ���Ͷ�����̼����ʯ��ˮ�У��Ȼ�������ˮ�γ����ᣬ�����ܺ��������Ʒ�Ӧ��Ҳ�ܹ������ɵ�̼��Ʒ�Ӧ�����Բ��ܲ���������
���Ũ������лӷ��ԣ����������Ʒ�Ӧ����Ũ�����лӷ��ԣ������ij����������ܽ⣩��
����������ij�����ʵ���������ʱ��ע�ⲻҪ©����100%����������ʱ�������Եģ�
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������к����������ҹ������涨����2011��5��1���𣬹������ڽ�ֹ���̣��̲�ȼ���ͷŵ������У�����Ѫ�쵰��������ж����ǣ�������
A��һ����̼ B��������̼ C����Ŷ� D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ʯ����Һ��ϡ�����У����������������������Һ��ʯ����ɫ�仯�Ĺ����ǣ�������
A����������� B���ϡ������� C�������ϡ��� D������ϡ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ�������ͼ����������ϵ�һ���ǣ�������
A��

��һ���¶���ij�����Ȼ�����Һ�м����Ȼ��ع���
B��

���������þ��п�м���ϡ����
C��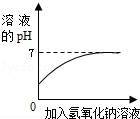

��ϡ�����м�������������Һ
D��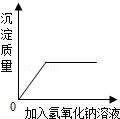

��������Ȼ����Ļ����Һ�м�������������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ����ĩ���������Ȼ�����̼���ơ�������þ���Ȼ����������ƺ�������е�ij������ɣ�Ϊ�˼�����ɷ֣�ʵ�����£�
��1����������ĩ��������ˮ�У����衢���á����ˡ��ð�ɫ��������ɫ��Һ��
��2�������ó����м�������ϡ���ᣬ����ȫ���ܽ⣬�������������
��3������Һ�еμ���������Һ���а�ɫ�������ɣ���������ϡ���ᣬ�������ܽ⣮
�Ը������������ƶϸð�ɫ��ĩ��һ������������һ��û�����������ܺ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
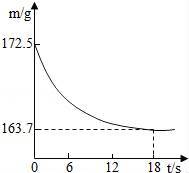
ij��˾�������Ĵ����Ʒ�о����ֻ�����Ȼ������ʣ�Ϊ�ⶨ��Ʒ��̼���Ƶ�����������20��ʱ����ȡ�ò�Ʒ��Ʒ26.5g�����뵽ʢ��һ������ϡ������ձ��У�̼������ϡ����ǡ����ȫ��Ӧ��������ȫ�ݳ����õ�������NaCl��Һ����Ӧ�����þ�����������ձ��ڻ�����������m���뷴Ӧʱ�䣨t����ϵ����ͼ��ʾ��
��
��1������CO2��������
��2���ô�����Ʒ��Na2CO3������������
��3���������������������������е�������ֻ����ĸ��ţ������㣬�ɶ�ѡ����
A���ô�����Ʒ��NaCl������������ B����Ӧ����ϡ�������������������
C����Ӧ��������Һ��NaCl������������ D����Ӧ����ˮ��������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ȵ�a��b��������ֱ������������������ͬ������ϡ�����г�ַ�Ӧ�����ɵ�������������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ��������a��b����Ԫ�صĻ��ϼ�j��Ϊ+2�ۣ����������й��������ܴ�����ǣ�������


A�������Ļ��ǿ����a��b
B������H2�������ǣ�a��b
C����Է��������ǣ�a��b
D����ȫ��Ӧ��������Һ��������a��b
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A��B��C��D��֧�Թ��зֱ�ʢ��CuSO4��NaCl��BaCl2��BaCO3���ְ�ɫ���壬ijͬѧ��ֻ������ˮ�������Թܵ������£�������һһ���������
��1������һ��ȡ���ֹ�����������ֱ����4֧�Թ��У�������������ˮ��˫�죮�������ʵ��ܽ��Լ���Һ����ɫ�ɼ����������
��2���������ǩ���������ʣ�����������������������Ϊ���йط�Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼΪij��Ƭ����Ʒ��ǩ������ݱ�ǩ���й���Ϣ������и��⣺
��1����Ҫ�ɷ�̼��ƣ�CaCO3���и�Ԫ�ص�����������Ca%��=�� ��100%=40%������д���ⲽ�裩��
��100%=40%������д���ⲽ�裩��
��2��ij��ͯ����ǩ������ҩ��ÿ�첹���Ԫ����������������д���ⲽ�裩��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com