
| 1g������ȫȼ�ղ��� | |||
| �ų�������/kJ | CO2������/g | ����SO2������/mg | |
| ���� | 56 | 2.75 | 0.3 |
| ú | 32 | 3.67 | 11 |
���� ��1�����ݻ�ʯȼ�ϵ����������
��2�����ݼ���ȼ�����ɶ�����̼��ˮ��д����Ӧ�Ļ�ѧ����ʽ���ɣ�
��3��������Ŀ�ṩ����Ϣ�������
��� �⣺��1����ʯȼ�ϰ���ú����Ȼ����ʯ�ͣ���ʯȼ�����ڲ���������Դ��
��2������ȼ�����ɶ�����̼��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��CH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O��
��3������Ŀ�ṩ����Ϣ��֪����ͬ�����ļ����ú��ȣ�����ȼ�ղ����Ķ�����̼�٣��ų��������ࣻ
�ʴ�Ϊ����1��ʯ�ͣ�������������2��CH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O����3�������Ķ�����̼�٣��ų��������࣮
���� �����ѶȲ����˽⻯ʯȼ�ϵ����ࡢ��Ȼ����ȼ�ϵ��ŵ㡢��ѧ����ʽ����д��������ȷ�����Ĺؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ������ȼ������̿�ڵ�ʵ�� | B�� |  ����Ҷ����ǩ | ||
| C�� |  ����Ĥ������������ | D�� | 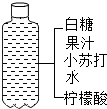 ������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������ĸA��H�������л��г��������ʣ��������⡢̼�������ơ��ơ����е�2-3��Ԫ�����
������ĸA��H�������л��г��������ʣ��������⡢̼�������ơ��ơ����е�2-3��Ԫ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѧ����Ϊ�����ṩ�²��� | B�� | ��ѧ���ڻ��������з�����Ҫ���� | ||
| C�� | ��ѧ����Ϊ�����ṩ��Դ | D�� | ��ѧ�ķ�չ��Ȼ������̬������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
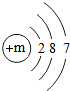 ��ͼΪijԪ�ص�ԭ�ӽṹʾ��ͼ����ԭ�ӵĺ���������m=17������Ԫ�����ڷǽ���Ԫ�أ���������ǽ����������ڻ�ѧ��Ӧ�����õ����ӣ���õ�����ʧȥ����
��ͼΪijԪ�ص�ԭ�ӽṹʾ��ͼ����ԭ�ӵĺ���������m=17������Ԫ�����ڷǽ���Ԫ�أ���������ǽ����������ڻ�ѧ��Ӧ�����õ����ӣ���õ�����ʧȥ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼΪijƷ��̼������Ƭ�ı�ǩ��ҽ����ʾ�����ø�ҩƷ����������NaCl�������������Ѫѹ���ߣ�������ձ�ǩ��ʾ����������ø�ҩ��ҩƷ��70%��θ�ᷴӦ�������ø�ҩһ����������NaCl�������������0.1g��
��ͼΪijƷ��̼������Ƭ�ı�ǩ��ҽ����ʾ�����ø�ҩƷ����������NaCl�������������Ѫѹ���ߣ�������ձ�ǩ��ʾ����������ø�ҩ��ҩƷ��70%��θ�ᷴӦ�������ø�ҩһ����������NaCl�������������0.1g���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �� | B�� | �� | C�� | �� | D�� | ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
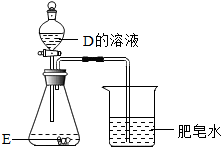
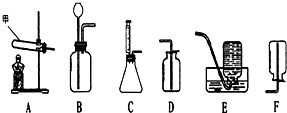 ����������ṩ��װ��ͼ�ش����⣺
����������ṩ��װ��ͼ�ش����⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com