
| ʵ����� | ʵ��Ŀ�� | ʵ ����� | ʵ������ | |
I | ��֤̼�� ������ | ����װ�м��� �ǵ�A�Թ��� ���� | ��B�Թ���Ӧ ������Լ��� | A�Թ��� B�Թ�����Һ��� �� |
�� | ��֤������ | ��ʵ���Ӧ���A�Թ��еμ� Na2C03��Һ | A�Թ�����Һ��� �� | |




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
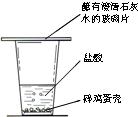 20����������֪���������ǵ���Ҫ�ɷ���̼��ƣ�ijͬѧΪ����֤�����ǵ���Ҫ�ɷ���̼��ƣ��������ͼ��ʵ�飮
20����������֪���������ǵ���Ҫ�ɷ���̼��ƣ�ijͬѧΪ����֤�����ǵ���Ҫ�ɷ���̼��ƣ��������ͼ��ʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 18����������֪���������ǵ���Ҫ�ɷ���̼��ƣ�ijͬѧΪ����֤�����ǵ���Ҫ�ɷ���̼��ƣ��������ͼ��ʵ�飮
18����������֪���������ǵ���Ҫ�ɷ���̼��ƣ�ijͬѧΪ����֤�����ǵ���Ҫ�ɷ���̼��ƣ��������ͼ��ʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 6����������֪���������ǵ���Ҫ�ɷ���̼��ƣ�ijͬѧΪ����֤�����ǵ���Ҫ�ɷ���̼��ƣ��������ͼ��ʵ�飮
6����������֪���������ǵ���Ҫ�ɷ���̼��ƣ�ijͬѧΪ����֤�����ǵ���Ҫ�ɷ���̼��ƣ��������ͼ��ʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2007?������Ϊ����֤�����ǵ���Ҫ�ɷ���CaCO3��ʢ��ͬѧ����������ʵ��̽����
��2007?������Ϊ����֤�����ǵ���Ҫ�ɷ���CaCO3��ʢ��ͬѧ����������ʵ��̽����| ʵ����� | ʵ��Ŀ�� | ʵ ����� | ʵ������ | |
I |
��֤̼�� ������ |
����װ�м��� �ǵ�A�Թ��� ���� ϡ���� ϡ���� |
��B�Թ���Ӧ ������Լ��� ����ʯ��ˮ ����ʯ��ˮ |
A�Թ��� �����DZ��������ݲ��� �����DZ��������ݲ��� B�Թ�����Һ��� �� |
�� |
��֤������ |
��ʵ���Ӧ���A�Թ��еμ� Na2C03��Һ |
A�Թ�����Һ��� �� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ɽ ���ͣ��ʴ���
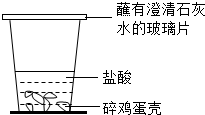
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com