


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ||
| ||
| ||

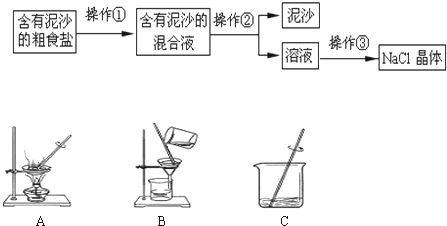
| Ń”ŌńµÄ×°ÖĆ | ŹµŃéĻÖĻó | ŹµŃé½įĀŪ |
AB AB |
BÖŠ³ĪĒåŹÆ»ŅĖ®²»±ä»ė×Ē BÖŠ³ĪĒåŹÆ»ŅĖ®²»±ä»ė×Ē |
ѳʷ²»ŗ¬¾§ĢåA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 NH3”ü+HCl”ü£®
NH3”ü+HCl”ü£®
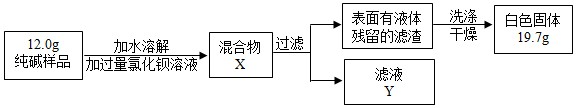
| Ń”ŌńµÄ×°ÖĆ | ŹµŃéĻÖĻó | ŹµŃé½įĀŪ |
| ______ | ______ | ѳʷ²»ŗ¬¾§ĢåA |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
ŠĖȤŠ”×é²Ī¹ŪijÖĘ¼ī³§ŗ󣬻ńµĆŅŌĻĀŠÅĻ¢²¢¶ŌĻą¹ŲĪŹĢā½ųŠŠŃŠ¾æ£®
”¾²éŌÄ׏ĮĻ”æ
¢Ł“ÖŃĪÖŠŗ¬ÓŠÉŁĮææÉČÜŠŌŌÓÖŹ£ØMgCl2ŗĶCaCl2£©¼°²»ČÜŠŌŌÓÖŹ£®
¢Ś·“Ó¦ŌĄķ£ŗNaCl£Ø±„ŗĶ£©+NH3+CO2+H2O=NaHCO3”ż+NH4Cl£¬½«·ÖĄė³öµÄ¾§ĢåA³ä·Ö¼ÓČČ£¬æÉÖʵƓæ¼ī£®
¢ŪNH4Cl NH3”ü+HCl”ü£®
NH3”ü+HCl”ü£®
¢ÜĪŽĖ®ĮņĖįĶÓöĖ®±äĄ¶
¢Ż²æ·ÖÉś²śĮ÷³ĢČēĻĀĶ¼ĖłŹ¾£ŗ

”¾ĪŹĢāĢÖĀŪ”æ
£Ø1£©¢ŁŠ“³ö¼ÓČėĒāŃõ»ÆÄĘČÜŅŗĖł·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½””£»””£®
¢Ś²Ł×÷¢ņµÄĆū³ĘĪŖ””£®
¢Ū·“Ó¦£Ø1£©ÖŠ¼ÓŹŹĮæŃĪĖįµÄ×÷ÓĆŹĒ””£®
¢Ü·“Ó¦£Ø2£©ÖŠĪŖĢįøß²śĀŹ£¬Ėł¼ÓĘųĢåµÄĖ³ŠņŹĒ””””£ØĢī×ÖÄø£©£®
A£®ĻČĶØČė¶žŃõ»ÆĢ¼ŌŁĶØ°±Ęų B£®ĻČĶØČė°±ĘųŌŁĶضžŃõ»ÆĢ¼
£Ø2£©ÉĻŹöÉś²śĮ÷³ĢÖŠ²»æÉŃ»·Ź¹ÓƵďĒ””””£ØĢī×ÖÄø£©£®
A£®CO2 B£®NH3 C£®HCl D£®NaOH
”¾×é³ÉĢ½¾æŅ»”æ
£Ø3£©¢Ł¾§ĢåAŹÜČČ·Ö½āµÄ»Æѧ·½³ĢŹ½ĪŖ””£»””£®
¢ŚÉč¼ĘŹµŃé¼ģŃé“æ¼īѳʷ֊ŹĒ·ń»ģÓŠ¾§ĢåAĒėĶź³ÉĻĀ±ķ£ŗ

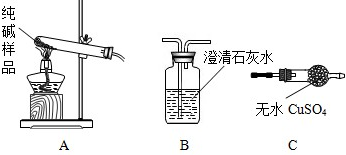
| Ń”ŌńµÄ×°ÖĆ | ŹµŃéĻÖĻó | ŹµŃé½įĀŪ |
| ”””” | ”” | ѳʷ²»ŗ¬¾§ĢåA |
”¾×é³ÉĢ½¾æ¶ž”æ
£Ø4£©Č”“æ¼īѳʷ¼ÓĖ®Čܽā£¬ĻņĘäÖŠ¼ÓČė¹żĮæĻ”HNO3£¬ŌŁµĪ¼ÓAgNO3ČÜŅŗ£¬ÓŠ°×É«³Įµķ£®²śÉś³ĮµķµÄ·½³ĢŹ½ĪŖ””£¬Č·¶Ø“æ¼īѳʷŗ¬ÓŠŌÓÖŹNaCl£®
”¾×é³ÉĢ½¾æČż”æ
£Ø5£©Ķ¬Ń§ĆĒĪŖĮĖ²ā¶ØøĆ“æ¼īѳʷµÄ“æ¶Č£¬Éč¼ĘĮĖČēĻĀŹµŃé£ŗ

¢ŁÅŠ¶Ļ¼ÓČėBaCl2ČÜŅŗŹĒ·ń¹żĮæµÄŗĻŹŹ·½·ØŹĒ””””£¬¹Ū²ģĻÖĻóÅŠ¶Ļ£®
A£®¾²ÖĆ»ģŗĻĪļX£¬ĻņÉĻ²ćĒåŅŗÖŠŌŁµĪBaCl2ČÜŅŗ
B£®ĻņĀĖŅŗYÖŠµĪ¼ÓBaCl2ČÜŅŗ
¢ŚÅŠ¶ĻĀĖŌüŹĒ·ńĻ“µÓøɾ»£¬æɲÉČ”ĻņĻ“µÓŅŗÖŠµĪ¼Ó””””£¬¹Ū²ģĻÖĻóÅŠ¶Ļ£®
A£®BaCl2ČÜŅŗ B£®Ļ”H2SO4 C£®Na2CO3ČÜŅŗ D£®Ļ”HCl
¢Ūøł¾ŻŹµŃ鏿¾Ż£¬¼ĘĖćѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹżĪŖ”” £ØŠ“³ö¼ĘĖć¹ż³Ģ£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2013Äź½ĖÕŹ”Õņ½ŹŠ¾ÅÄź¼¶ĶųÉĻŌľķŹŹÓ¦ŠŌѵĮ·»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
 NH3”ü+HCl”ü£®
NH3”ü+HCl”ü£®

| Ń”ŌńµÄ×°ÖĆ | ŹµŃéĻÖĻó | ŹµŃé½įĀŪ |
| ѳʷ²»ŗ¬¾§ĢåA |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2013Äź½ĖÕŹ”Õņ½ŹŠÖŠæ¼»ÆѧŅ»Ä£ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
 NH3”ü+HCl”ü£®
NH3”ü+HCl”ü£®

| Ń”ŌńµÄ×°ÖĆ | ŹµŃéĻÖĻó | ŹµŃé½įĀŪ |
| ѳʷ²»ŗ¬¾§ĢåA |

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com