
 ����ͭ��þ���ֽ�����ɵĻ������ⶨ����ɣ���ȡ�û�����ĩ8g�����ձ��У���140gϡ����ƽ�����Ĵμ�����ձ��У���ַ�Ӧ���ʣ������������ݼ�¼��ͼ��ͨ�����㣬��
����ͭ��þ���ֽ�����ɵĻ������ⶨ����ɣ���ȡ�û�����ĩ8g�����ձ��У���140gϡ����ƽ�����Ĵμ�����ձ��У���ַ�Ӧ���ʣ������������ݼ�¼��ͼ��ͨ�����㣬��
���� ��1����ͭ�����Ӧ����Ӧ��ʣ���������4.2g��Ϊ���������ʿ����������ĩ��ͭ������������
��2������ǰ���η�Ӧ����ϡ������ȫ��Ӧʱ�������������������ϵ�����ݷ�Ӧ�Ļ�ѧ����ʽ�����������������ȷ���������������������
��3�����ݷ�Ӧ�Ļ�ѧ����ʽ���ɷ�Ӧ�����������������ų����������������������غ㶨�ɣ����㷴Ӧ��������Һ��������
��4������ϡ��Ũ����IJ�����з�����
��� �⣺��1����ͭ�����Ӧ����Ӧ��ʣ���������4.2g��Ϊ��������$\frac{4.2g}{8.0g}$��100%=52.5%
�ʴ�Ϊ��52.5%
��2���ɵ�һ������ϡ����140g��4=35g��ȫ��Ӧʱ������þ������=8.0g-6.8g=1.2g��
��μӷ�Ӧ����������Ϊx����
Mg+H2SO4�TMgSO4+H2��
24 98
1.2g x
$\frac{24}{1.2g}$=$\frac{98}{x}$
x=4.9g��
���������������������$\frac{4.9g}{35g}$��100%=14%
�ʴ�Ϊ��14%��
��3�������μ��������һ��������35g��3=105g����
�����105gϡ�����ַ�Ӧ��������������Ϊx����������þ������Ϊy
Mg+H2SO4�TMgSO4+H2��
24 120 2
8.0g-4.4g y x
$\frac{24}{8.0g-4.4g}$=$\frac{120}{y}$=$\frac{2}{x}$
x=0.3g��y=18g
������Һ������=��8.0g-4.4g��+105g-0.3g=108.3g
�����ʵ���������Ϊ��$\frac{18g}{108.3g}$��100%=16.6%
��������Һ�����ʵ�����������16.6%
��4��ʵ������Ҫ��98%��Ũ����ϡ��Ϊ20%��ϡ���ᣬ��Ҫ�õ��������У���Ͳ����ͷ�ιܡ��ձ�������������ѡ�ܢߣ�
���� �����ѶȽϴ���Ҫ����ѧ������ͼ�����ݡ���ѧ����ʽ���������������ۺ�Ӧ�ý�����������������ÿ�μ���ϡ����ǰ������������Ӧ���Ľ�����������ϵ�жϼ���ϡ����ķ�Ӧ�������ȷ�����Ĺؼ���


 �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д� ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ҵ��ˮ���������ŷ� | B�� | ��ֹʹ��ũҩ�ͻ��� | ||
| C�� | �����ˮ�������㵹���� | D�� | �������ú���ϴ�·� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��˿�������о���ȼ�գ������� | |
| B�� | ��Ũ������Լ�ƿ�ǣ�ƿ�ڻ���ְ��� | |
| C�� | �ڵ��ˮʵ���У���Դ����������������������Ϊ2��1 | |
| D�� | ������������������ⶨʵ��������뼯��ƿ��ˮ�����ԼΪ����ƿ�����������$\frac{1}{5}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ���Ƕ���ֱ�ӹ������� | B�� | ���Ƕ��DZ������ʻ�ѧ���ʵ����� | ||
| C�� | ���Ƕ��ǵ����Ե����� | D�� | ���Ƕ����ڲ�ͣ���˶� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
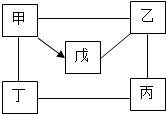 �ס��ҡ������������Ϊ���л�ѧ�������ʣ���ͼ��ӳ������֮������ϵ�����С�-����ʾ���������ʼ�������Ӧ����������ʾ����һ�������¿���ת��Ϊ�죮����Ӧ��������������������ȥ����ش�
�ס��ҡ������������Ϊ���л�ѧ�������ʣ���ͼ��ӳ������֮������ϵ�����С�-����ʾ���������ʼ�������Ӧ����������ʾ����һ�������¿���ת��Ϊ�죮����Ӧ��������������������ȥ����ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������Һ�У�Al3+��Cu2+��OH- | B�� | ������������Һ�У�H+��Ca2+��Cl- | ||
| C�� | ���Ȼ�����Һ�У�Na+��K+��NO3- | D�� | ����������Һ�У�Ba2+��Cl-��OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com