
(8��)ˮ����Ҫ����Ȼ��Դ��
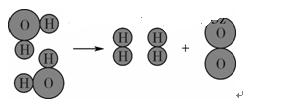
(1)��ͼ��ʾ��3��ʵ�飬A��ˮ������ (���������ѧ��)�仯��B���Թ�1�ڵõ�������Ϊ ��C�о���ˮ�ķ����� ��������

A��ˮ�ķ��� B��ˮ�ĵ�� C��ˮ�ľ���
(2) ʵ���ҵķ�Һ�辭���������ŷţ�Ϊ������������ķ�Һ�����ԣ�����ѡ�õ�������_______(����ĸ���)��
A�������� B����ʯ�� C��������̼ D��̼����
(3) ����ˮ����������ˮ�Ĺ������������ʯ�ң���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ ����2�֣�
(4)������(Na2FeO4)��һ�����;�ˮ��������������ɱ���������á��뽫������ȡ�����ƵĻ�ѧ����ʽ����������2Fe(NO3)3+16NaOH+3Cl2=6NaNO3+6NaCl+2Na2FeO4+ ����2�֣�
(1)���� H2 ���� (2) C (3) CaO+H2O=Ca(OH)2 (4)8H2O
����������1��Aʵ���������������ɣ������������仯��B���Թ�1�ڵõ����������������жϷ����ǣ�һ�������������߶������Թ����ӵ��Ǹ�����Cװ�õĻ���̿���������ԣ��������ʶ�ˮ�й��˺����������ã��ʴ�Ϊ��������������H2�����ˣ�
��2�����������ǰ���������̼���η�Ӧ���������̼����Ӧ����ѡC��
��3����ʯ����ˮ��Ӧ�����������ƣ��ʴ�Ϊ��CaO+H2O=Ca��OH��2��
��4����ѧ��Ӧǰ��ԭ�ӵ�����������䣬�ɴ˿�֪�÷�Ӧ�ķ���ʽ��2Fe��NO3��3+16NaOH+3Cl2=6NaNO3+6NaCl+2Na2FeO4+8H2O���ʴ�Ϊ��8H2O��


 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ˮ���� | B������� |
| C�������� | D����ԭ�Ӻ���ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����б�ҵ��ѧ���ԣ���������������ѧ ���ͣ������
(6��)ˮ����Ҫ����Ȼ��Դ��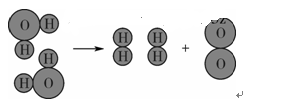
(1)ˮ�������������������˵��ˮ����________Ԫ�غ�________Ԫ����ɡ�
(2)��ͼ��ˮ���ӷֽ�ʾ��ͼ����һ��ѧ�仯�е���С������________(����)��
| A��ˮ���� | B������� |
| C�������� | D����ԭ�Ӻ���ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��㶫ʡï���о��꼶��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
(8��)ˮ����Ҫ����Ȼ��Դ��
(1)��ͼ��ʾ��3��ʵ�飬A��ˮ������ (���������ѧ��)�仯��B���Թ�1�ڵõ�������Ϊ ��C�о���ˮ�ķ����� ��������
A��ˮ�ķ��� B��ˮ�ĵ�� C��ˮ�ľ���
(2) ʵ���ҵķ�Һ�辭���������ŷţ�Ϊ������������ķ�Һ�����ԣ�����ѡ�õ�������_______(����ĸ���)��
A�������� B����ʯ�� C��������̼ D��̼����
(3) ����ˮ����������ˮ�Ĺ������������ʯ�ң���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ ����2�֣�
(4)������(Na2FeO4)��һ�����;�ˮ��������������ɱ���������á��뽫������ȡ�����ƵĻ�ѧ����ʽ����������2Fe(NO3)3+16NaOH+3Cl2=6NaNO3+6NaCl+2Na2FeO4+ ����2�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ�������µڶ�ѧУ���꼶��ѧ�����м�⻯ѧ�Ծ����������� ���ͣ������
(2��)ˮ����Ҫ����Ȼ��Դ��ˮ��Դ���㽫����Ӱ����������档
��1��Ϊ�˱��ֽ��Ӻ�����������ijͬѧ������������˱������ӵļ������飺
�����з������磬������̬ƽ�⣻ �����û��ú���ϴ�·ۣ� �۽�ֹ��ӵ����㵹������ �ܽ�ֹʹ�û��ʺ�ũҩ����ֹˮ����Ⱦ�����в��������� ������ţ���
��2��Ϊ��������ˮ��Դ���㻹��ʲô���飺 ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com