
·”ΔΒΡ÷ς“Σ≥…Ζ÷ΈΣΕΰ―θΜ·ΙηΘ§Υϋ «≤ΘΝßΙΛ“ΒΚΆΧ’¥…ΙΛ“ΒΒΡ‘≠ΝœΘ§“±ΫπΙΛ“ΒΒΡ÷ζ»έΦΝΓΘ
Θ®1Θ©Εΰ―θΜ·ΙηΒΡΫαΙΙ”κΫπΗ’ ·œύΥΤΘ§ΕΦ «”… Θ®ΧνΓΑΖ÷Ή”Γ±ΓΔΓΑ‘≠Ή”Γ±ΜρΓΑάκΉ”Γ±Θ©ΙΙ≥…ΒΡΘ§Εΰ―θΜ·Ιη Θ®ΧνΓΑ τ”ΎΓ±ΜρΓΑ≤Μ τ”ΎΓ±Θ©―θΜ·ΈοΓΘ
 Θ®2Θ©ΉΑ”–«β―θΜ·ΡΤ»ή“ΚΒΡ ‘ΦΝΤΩ≤ΜΡή”Ο≤ΘΝß»ϊΘ§‘≠“ρ «‘Ύ≥ΘΈ¬œ¬Θ§NaOH”κ≤ΘΝß»ϊ÷–ΒΡSiO2ΜΚ¬ΐΒΊΖΔ…ζΖ¥”Π…ζ≥…Na2SiO3ΚΆH2OΘ§Na2SiO3 ΙΤΩΩΎ”κΤΩ»ϊ’≥Κœ‘Ύ“ΜΤπΘ§‘ρΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ ΓΘ
Θ®2Θ©ΉΑ”–«β―θΜ·ΡΤ»ή“ΚΒΡ ‘ΦΝΤΩ≤ΜΡή”Ο≤ΘΝß»ϊΘ§‘≠“ρ «‘Ύ≥ΘΈ¬œ¬Θ§NaOH”κ≤ΘΝß»ϊ÷–ΒΡSiO2ΜΚ¬ΐΒΊΖΔ…ζΖ¥”Π…ζ≥…Na2SiO3ΚΆH2OΘ§Na2SiO3 ΙΤΩΩΎ”κΤΩ»ϊ’≥Κœ‘Ύ“ΜΤπΘ§‘ρΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ ΓΘ
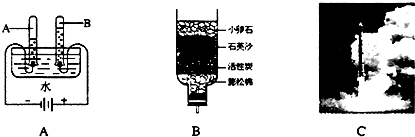
Θ®3Θ©Ιη «ΧΪ―τΡήΒγ≥ΊΚΆΒγΡ‘–ΨΤ§≤ΜΩ…»±…ΌΒΡ≤ΡΝœΓΘ…ζ≤ζΗΏ¥ΩΙηΒΡΝς≥Χ Ψ“βΆΦ»γœ¬ΘΚ
|
ΔΌ ÷Τ±Η¥÷ΙηΒΡΖ¥”ΠΈΣΘΚSiO2 + 2C=== Si + 2COΓϋΓΘ
ΔΎ ’ϊΗω÷Τ±ΗΙΐ≥Χ±Ί–κ¥οΒΫΈόΥ°Έό―θΘ§»τ‘ΎH2ΜΙ‘≠SiHCl3Ιΐ≥Χ÷–Μλ»κO2Θ§Ω…Ρή“ΐΤπΒΡΚσΙϊ « ΓΘ
Δέ ΈΣΝΥ¥οΒΫ¬Χ…ΪΜ·―ßΚΆΫΎ‘ΦΉ ‘¥ΒΡΡΩΒΡΘ§Έο÷ A–η“Σ―≠ΜΖ Ι”ΟΘ§AΒΡΜ·―ß Ϋ « ΓΘ

| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
| ||
| ||
| ||
| ||
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com