


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013����б�ҵ��ѧ���ԣ��������ž�����ѧ�������棩 ���ͣ������
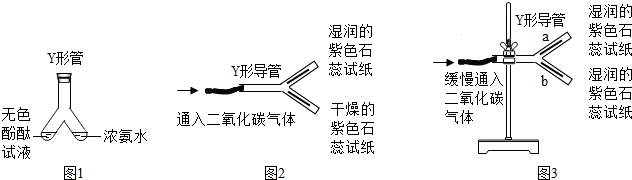
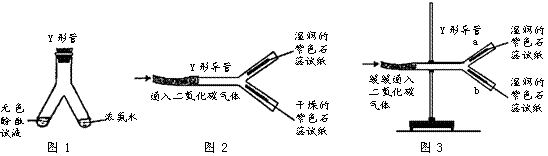
�� Y�ιܻ� Y �ε����������ʵ�顣
��1��ͼ9��Y�ι������м�����ɫ��̪��Һ���Ҳ���м���Ũ��ˮ��һ��ʱ��ɹ۲쵽������________________________���÷��ӵĹ۵������һ����________________________��
��2��ͼ10 �� Y �ε���ƽ�������棬ʵ���пɹ۲쵽������________________________���û�ѧ����ʽ���ͽ��ۣ�________________________������Y�ε��̶ܹ�������̨�ϣ���ͼ 11 ) , a ��λ���Ϸ��� b ��λ���·�����ʯ����ֽ��ʪ�ɹ۲쵽 b ������ֽ��ɫ��a �����ԣ�ԭ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
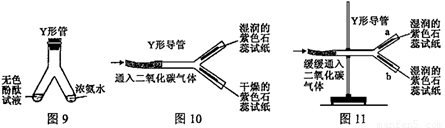
�� Y �ιܻ� Y �ε����������ʵ�顣
��1��ͼ 9 �� Y �ι������м�����ɫ��̪��Һ���Ҳ���м���Ũ��ˮ��һ��ʱ��ɹ۲쵽������________________________���÷��ӵĹ۵������һ����________________________��
��2��ͼ 10 �� Y �ε���ƽ�������棬ʵ���пɹ۲쵽������________________________���û�ѧ����ʽ���ͽ��ۣ�________________________������ Y �ε��̶ܹ�������̨�ϣ���ͼ 11 ) , a ��λ���Ϸ��� b ��λ���·�����ʯ����ֽ��ʪ�ɹ۲쵽 b ������ֽ��ɫ�� a �����ԣ�ԭ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
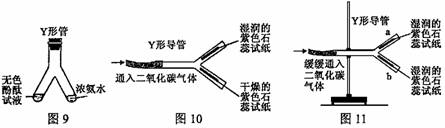
�� Y �ιܻ� Y �ε����������ʵ�顣
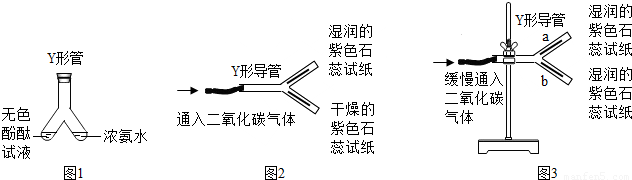
�� ͼ 1�� Y �ι������м�����ɫ��̪��Һ���Ҳ���м���Ũ��ˮ��һ��ʱ��ɹ۲쵽������ ���÷��ӵĹ۵����һ��������ǣ� ��
�� ͼ2��Y�ε���ƽ�������棬ʵ���пɹ۲쵽������ ��
���÷��ű���ʽ���ͽ��ۣ� ������ Y �ε��̶ܹ�������̨�ϣ���ͼ 3 ) , a ��λ���Ϸ��� b ��λ���·�����ʯ����ֽ��ʪ�ɹ۲쵽 b ������ֽ��ɫ�� a �����ԣ�ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�긣��ʡ�������п���ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com