



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
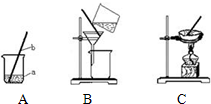 ��1���������γ����ᴿ��ʵ��ʱ��Ҫ������ͼ��ʾ��ʵ�������
��1���������γ����ᴿ��ʵ��ʱ��Ҫ������ͼ��ʾ��ʵ�������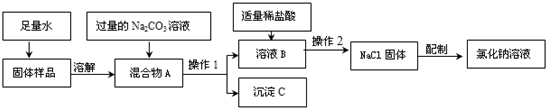
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ����������� | �����������+ ʳ����Һ������ |
�����������+ ʳ�ξ�������� |
| 28.8g | 48.8g | 30.7g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�020
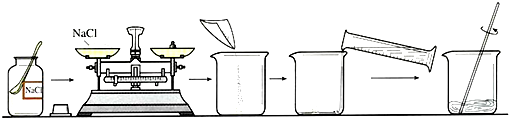
ʵ��������һ�����ʵ�����������ʳ����Һ���ɿˣ���Ҫһ������ʳ�Σ����ú������Ե����ʵĴ��������ƣ��辭���¼���������
��ϴ�Ӹ��� �ڹ��� ���ܽ� �ܳ��� �������ᾧ
��ȷ�IJ���˳��Ӧ�ǡ�����������
[����]
A.�ۢڢ٢ܢݡ��������� B.�ۢݢڢ٢ܢۡ�����
C.�ۢڢݢ٢ܢۡ������� ��D.�٢ۢڢݢ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com