
 2KCl+3O2↑
2KCl+3O2↑ =
=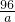
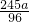
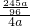 ×100%=63.8%
×100%=63.8%

 阅读快车系列答案
阅读快车系列答案科目:初中化学 来源: 题型:
查看答案和解析>>
科目:初中化学 来源:不详 题型:问答题
查看答案和解析>>
科目:初中化学 来源: 题型:
小英同学为了制取氧气,取一定量的KClO3和一定量的MnO2混合共热,开始时MnO2在混合物中的质量分数为20%,当MnO2的质量分数提高到25%时,试计算KClO3分解的质量分数。(提示:混合物中某物质的质量分数,就是该物质的质量与混合物总质量之比。)
查看答案和解析>>
科目:初中化学 来源:2009-2010学年新人教版九年级(下)期末化学试卷(2)(解析版) 题型:解答题
查看答案和解析>>
科目:初中化学 来源:2008-2009学年江苏省无锡市宜兴市九年级(上)期末化学试卷(解析版) 题型:解答题
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com