

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013����б�ҵ��ѧ���ԣ��㶫���ݾ�����ѧ���������� ���ͣ��ʴ���
ʵ�����е��Լ�һ��Ҫ�ܷⱣ�棬������ܻ�������Ӵ������ʡ�ij�о���ѧϰС�鷢��һƿδ�ܱյ�KOH���壬����ɷ�������¼��裬�������ʵ��̽����
����1��ֻ��KOH�� ����2����KOH��K2CO3�� ����3��ֻ��K2CO3
��1���ɷ��п��ܺ���K2CO3��ԭ���ǣ��û�ѧ����ʽ�ش�________________________��
��2��ȡ������Ʒ���Թ��У���������ϡ���ᣬ�۲쵽_____________________��˵������2�����3������
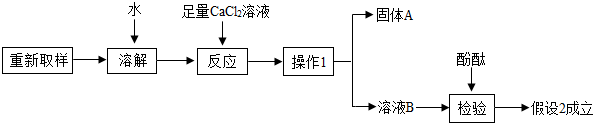
��3����һ��̽���Ĺ������£� 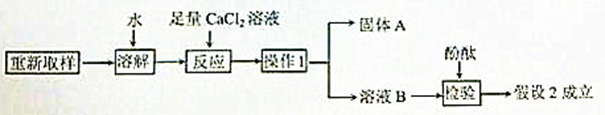
�١�����1����������____________��
�ڡ�����A���Ļ�ѧʽ��___________��
�ۼ�������CaCl2��Һ��������_______________________________��
��4�������Լ�Ҳ�����ü�ֵ����KOH��K2CO3����ɺ����ʿ�����ƿ�����Լ�����;��
__________________________________________��д��һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013����б�ҵ��ѧ���ԣ��㶫���ݾ�����ѧ�������棩 ���ͣ������
ʵ�����е��Լ�һ��Ҫ�ܷⱣ�棬������ܻ�������Ӵ������ʡ�ij�о���ѧϰС�鷢��һƿδ�ܱյ�KOH���壬����ɷ�������¼��裬�������ʵ��̽����
����1��ֻ��KOH�� ����2����KOH��K2CO3�� ����3��ֻ��K2CO3
��1���ɷ��п��ܺ���K2CO3��ԭ���ǣ��û�ѧ����ʽ�ش�________________________��
��2��ȡ������Ʒ���Թ��У���������ϡ���ᣬ�۲쵽_____________________��˵������2�����3������
��3����һ��̽���Ĺ������£�
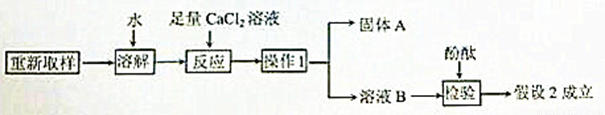
�١�����1����������____________��
�ڡ�����A���Ļ�ѧʽ��___________��
�ۼ�������CaCl2��Һ��������_______________________________��
��4�������Լ�Ҳ�����ü�ֵ����KOH��K2CO3����ɺ����ʿ�����ƿ�����Լ�����;��
__________________________________________��д��һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ�������п���ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com