
 �Ķ�������ն��ģ��������������¸�д����
�Ķ�������ն��ģ��������������¸�д�������� ��1��ʳ���ɻ���ʱ������������֭���Գ�ȥ�ɻ����еļ�ɬζ��
��2���������������л�����ǿ��NaOH��KOH����Ԫ��������ԭ���еIJ�ľ�ң�
�����ƺ�ˮ��Ӧ�����������ƣ����ڻ��Ϸ�Ӧ���������ƺ�̼���Ʒ�Ӧ����̼��Ƴ������������ƣ����ڸ��ֽⷴӦ��
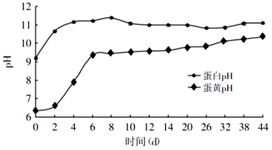
��3�������ɻ�������ʱ�������е���͵���pH�ı仯��ϵ�����жϣ�������͵���pH
���ﵽ9����ʱ���ɻ����������Ƶ�������
��4���ɻ���������ɫ���γ������������йأ�
�����ɻ������ܺ���������Ǧ����ͯ����ʳ�ã�
��� �⣺��1��ʳ���ɻ���ʱ������������֭���Գ�ȥ�ɻ����м�ɬζ��
�����ɬ��
��2���������������л�����ǿ��NaOH��KOH����Ԫ��������ԭ���еIJ�ľ�ң�
����NaOH�Ĺ����У����ڸ��ֽⷴӦ�����������ƺ�̼���Ʒ�Ӧ����̼��ƺ��������ƣ���Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+Ca��OH��2�TCaCO3��+2NaOH��
�����ľ�ң�Na2CO3+Ca��OH��2�TCaCO3��+2NaOH��
��3�������ɻ�������ʱ�������е���͵���pH�ı仯��ϵ�жϣ�������͵���pH���ﵽ9����ʱ���ɻ����������Ƶ�����Ϊ6�죮
��ѡ��B��
��4���ɻ���������ɫ���γ������������йأ�
�����ɻ������ܺ���������Ǧ����ͯ����ʳ�ã�
��ѡ��AC��
���� ��ѧ��Դ���������Ҳ������������������������������������صĻ�ѧ֪ʶҲ����Ҫ���п��ȵ�֮һ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ̼-12ԭ�Ӻ�̼-13ԭ�ӵ���������ͬ | |
| B�� | KMnO4��K2MnO4������ͬ��ԭ���� | |
| C�� | Fe2+��Fe3+��������Ԫ�أ������������ͬ | |
| D�� | 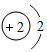 �� ��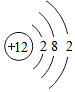 ��ѧ������ͬ ��ѧ������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������Ϊ������ɫ�����ṩ�˱��� | |
| B�� | ���á��������͡�������Ԥ��ȱ����ƶѪ | |
| C�� | �û�ѧ���ϸ�װ�õķ��ӣ����ͷų���ȩ�����ʣ�����������ס | |
| D�� | �ѺϽ���ʴ��ǿ����Ӧ���ڡ������š�DZˮ���ϣ��ѺϽ������л��ϳɲ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A����ѧ�뽡�� | B����ѧ����Դ |
| ��������ȱ�ƻᵼ�¹������� ������ȱ��ά����A������ҹä֢ ��Ҫ�μǡ������к�����������Ʒ�����Բ��ã��� | ��̫���ܡ����ܺ���Ȼ������������Դ �ڵ�س��ʱ����ѧ��ת��Ϊ���� �ۻ�ʯȼ�������ʵ���Դ��Դ��������Ϊ������Դ |
| C����������� | D����ѧ�뻷�� |
| ����Ȼ���ͺ�ˮ�����ڻ����� ���ռ�ʹ�����ڼ� ������[CO��NH2��2]������أ�KNO3�������ڸ��� �� | ��ʩ�ù������ʡ�ũҩ������ʳ���� ��ʹ�ÿɽ����������Ʒ������ �۵�̼������о������л������г� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʯ��ˮ | B�� | ���� | C�� | ���ʯ | D�� | ʯ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | X | O2 | CO2 | H2O |
| ��Ӧǰ����/g | 23 | 70 | 1 | 0 |
| ��Ӧ������/g | 0 | ���� | 45 | 27 |
| A�� | �÷�ӦΪ�ֽⷴӦ | |
| B�� | ��Ӧ���ɵ�CO2��H2O��������Ϊ45��27 | |
| C�� | ���С����⡱ֵΪ48 | |
| D�� | X��һ������̼���⡢������Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������� | B�� | ����ë�� | C�� | �����Ʊ� | D�� | �������ͷ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com