
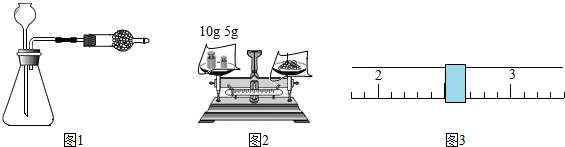
| ʱ�䣨s�� | [11��28�� | [28��47�� | [47��62�� | [62��74�� | [74��88�� | [88��103�� | [103��118�� | [118��135�� | [135��154�� | [154��176�� |
| ������g�� | 98.0 | 97.9 | 97.8 | 97.7 | 97.6 | 97.5 | 97.4 | 97.3 | 97.2 | 97.1 |
| 100 |
| x |
| 44 |
| 2.2g |
| 12.5g��80%-5g |
| 12.5g-5g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ʳ�η���ˮ�г�ֽ��� |
| B����ʯ�ҷ���ˮ�г�ֽ��� |
| C����ͭ�Ƴɱ�������ͭ�� |
| D����Ũ�����ˮϡ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ΪϡBa��NO3��2��Һ����ΪAgNO3��Һ����ΪHNO3��Һ |
| B����ΪϡBa��NO3��2��Һ����ΪNaNO3��Һ����ΪHNO3��Һ |
| C����ΪϡHNO3��Һ����ΪAgNO3��Һ����ΪNaNO3��Һ |
| D����ΪϡNaNO3��Һ����ΪHNO3��Һ����ΪAgNO3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
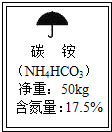 ͼΪij���ʰ�װ���ϵı�ǩ��С��ͨ��������������õ����е��ĺ�������װ��ϡ�����С�ձ���������Ϊ200��Og������16.0g�õ��ʣ���ֽ��裬����ʣ�࣮��Ӧ��С�ձ�����Һ������Ϊ207.2g������֪��NH4HC03+HCl=NH4Cl+H20+C02�� ��
ͼΪij���ʰ�װ���ϵı�ǩ��С��ͨ��������������õ����е��ĺ�������װ��ϡ�����С�ձ���������Ϊ200��Og������16.0g�õ��ʣ���ֽ��裬����ʣ�࣮��Ӧ��С�ձ�����Һ������Ϊ207.2g������֪��NH4HC03+HCl=NH4Cl+H20+C02�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
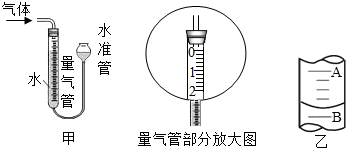
| ��� | KClO3������g�� | ��������������g�� | �������� |
| 1 | 2.0 | ��������� | �� |
| 2 | 2.0 | CuO 0.5 | �� |
| 3 | 2.0 | MnO2 0.5 | �� |
| ʵ�鲽�� | ʵ����̼����� | ���Ŀ�� |
| 1 | ����CuO�����O2�����ʱ�δ����ʱ��ö� | CuO�ܼӿ�KClO3�ķֽ� |
| 2 | CuO�������ڷ�Ӧǰ���Ƿֲ��� | |
| 3 | �����˺��CuO������KClO3��ϼ��ȣ��۲��Ƿ������ܼӿ�KClO3�ķֽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
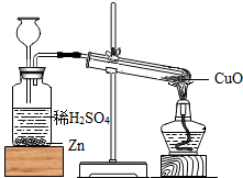
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
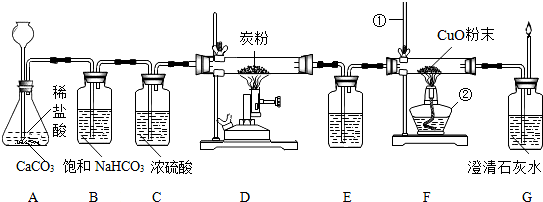
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com