
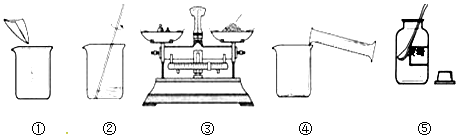
���� ��1����������=��Һ��������������������
��2����ͼ�١��ڡ�������ͬ�������������ձ���
ͼ���в������������Ǽ����ܽ⣻
��3����ȷ���Ƹ���Һ�IJ���˳��Ϊ��ȡ��ҩƷ������ҩƷ��������ҩƷ�����ձ��У���ȡˮ�����ձ��У����ò���������ʹҩƷ��ȫ�ܽ⣻
��4������ʱӦ��������������
��5��A������Ͳ��ȡˮʱ���Ӷ�ʱ���ᵼ��ˮ�����ƫ�Ӷ�����������Һ��������ƫС��
B��������Һ���ձ�����������ˮ��ϴ���ᵼ��ˮ�����ƫ�Ӷ�����������Һ��������ƫС��
C�����Ǿ��岻��ʱ���ᵼ����������ƫС���Ӷ�����������Һ��������ƫС��
D��ת������õ���Һʱ����������Һ��������Ӱ������������
��6�����ƺõ���ҺҪװ��ϸ��ƿ�в����ϱ�ǩ����ǩ��Ҫ������Һ�����ƺ���������������
��� �⣺��1������������Һ��С��Ҫ��ȡ��������Ϊ��100g��18.5%=18.5g��
���18.5��
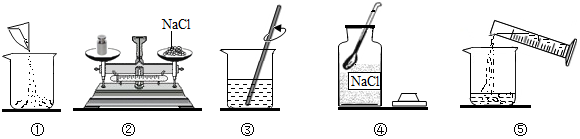
��2����ͼ�١��ڡ�������ͬ�������������ձ���
ͼ���в������������Ǽ����ܽ⣮
����ձ��������ܽ⣮
��3����ȷ���Ƹ���Һ�IJ���˳��Ϊ��ȡ��ҩƷ������ҩƷ��������ҩƷ�����ձ��У���ȡˮ�����ձ��У����ò���������ʹҩƷ��ȫ�ܽ⣮
����ݢۢ٢ܢڣ�
��4������ͼ�������������Ϊ15g������Ķ���Ϊ3.5g����С���Ƶõ���������ʵ��Ϊ��15g-3.5g=11.5g��
��������ķ����ǣ������Ƿŵ����̣�����ŵ����̣���������ֱ����ƽƽ�⣮
���11.5�������Ƿŵ����̣�����ŵ����̣���������ֱ����ƽƽ�⣮
��5��A������Ͳ��ȡˮʱ���Ӷ�ʱ���ᵼ��ˮ�����ƫ�Ӷ�����������Һ��������ƫС��
B��������Һ���ձ�����������ˮ��ϴ���ᵼ��ˮ�����ƫ�Ӷ�����������Һ��������ƫС��
C�����Ǿ��岻��ʱ���ᵼ����������ƫС���Ӷ�����������Һ��������ƫС��
D��ת������õ���Һʱ����������Һ��������Ӱ������������
���D��
��6�����ƺõ���ҺҪװ��ϸ��ƿ�в����ϱ�ǩ����ǩ��Ҫ������Һ�����ƺ���������������
���ϸ��ƿ����Һ�����ƺ���������������
���� �������ʵ�飬��ѧ�ؽ���ʵ�顢����ʵ�飬�ǵó���ȷʵ����۵�ǰ�ᣬ���Ҫѧ�����ʵ�顢����ʵ�顢����ʵ�飬Ϊѧ�û�ѧ֪ʶ�춨������


 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ֻ����̼��������Ԫ�� | B�� | ����̼���⡢������Ԫ�� | ||
| C�� | ֻ����̼������Ԫ�� | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �������綯��һ��ʹ��Ǧ�����أ�ijǦ������ʹ�õ�����Һ����������Ϊ20%��ϡ���ᣮ��ش������й����⣺
�������綯��һ��ʹ��Ǧ�����أ�ijǦ������ʹ�õ�����Һ����������Ϊ20%��ϡ���ᣮ��ش������й����⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ƹ��屣�治�����ѳ������ | |
| B�� | ��������ƽ�����������ƹ���ʱ��ҩƷ������λ�÷ŷ��� | |
| C�� | ��������ƽ������������ʱ��û�з��ڲ������г��� | |
| D�� | ����Ͳ��ȡˮʱ������ˮ��̶� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com