
”¾ĢāÄæ”æ“ÓŅŌĻĀĖł¹©ŅĒĘ÷ÖŠ£¬Ń”ŌńÉč¼Ę×ŌČ”¶žŃõ»ÆĢ¼µÄ×°ÖĆ£¬½ųŠŠ¶žŃõ»ÆĢ¼µÄÖĘČ”¼°ŠŌÖŹĢ½¾æ”£

(1)Öø³öĶ¼ÖŠŅĒĘ÷µÄĆū³Ę£ŗ¢Ś_____________£»
(2)ÖĘČ”²¢ŹÕ¼Æ¶žŃõ»ÆĢ¼£¬Ń”ÓĆÉĻĶ¼ŅĒĘ÷ÖŠµÄ_____________(ĢīŠņŗÅ)£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________________________£»
(3)ŹÕ¼ÆµÄŅ»Ę涞Ńõ»ÆĢ¼¼ģŃ鶞Ńõ»ÆĢ¼ŹĒ·ńŹÕ¼ÆĀśµÄ·½·ØŹĒ__________________________£»
(4)ĪŖĮĖĢ½¾æ¶žŃõ»ÆĢ¼ÄÜÓėĖ®·¢Éś·“Ӧɜ³É¾ßÓŠĖįŠŌµÄĪļÖŹ£¬¼×”¢ŅŅĮ½Ķ¬Ń§·Ö±šÉč¼ĘĮĖĢ½¾æŹµŃéµÄ·½°ø(ČēĶ¼¼×”¢ŅŅĖłŹ¾)½ųŠŠŹµŃ锣Ķ¼ŅŅ²ÉÓƵÄĖĶä»ØĪŖÓĆŹÆČļČÜŅŗČ¾³É×ĻÉ«µÄøÉŌļµÄÖ½»Ø”£

¢ŁĒėÄćÓė¼×”¢ŅŅĶ¬Ń§Ņ»ĘšĶź³ÉĢ½¾æ£ŗ
·½°øŅŅ | (¢ń) | (¢ņ) | (¢ó) | (¢ō) |
ĻÖĻó | ±äŗģÉ« | ²»±äÉ« | ²»±äÉ« | ±äŗģÉ« |
·ÖĪöĶ¼¼×”¢ŅŅÖŠCO2Ź¹ŹÆČļŹŌŅŗ±äŗģÉ«µÄŌŅņ_______________£»
¢ŚÄćČĻĪŖ¼×”¢ŅŅÄÄĪ»Ķ¬Ń§µÄŹµŃéĢ½¾æ·½°øŗĻĄķ£¬²¢Ėµ³öÄćµÄĄķÓÉ£ŗ_______________________”£
”¾“š°ø”æ׶ŠĪĘæ ¢Ś¢Ū¢Ü¢ß CaCO3+2HCl=CaCl2+CO2”ü+ H2O ½«Č¼×ŵÄľĢõĘ½·ÅÓŚĘææŚ£¬ČōľĢõĻØĆšŌņŅŃĀś ¶žŃõ»ÆĢ¼ÓėĖ®·“Ӧɜ³ÉĮĖĢ¼Ėį ŅŅĶ¬Ń§·½°øŗĻĄķ£»ŅŅĶ¬Ń§Ķعż¶Ō±ČŹµŃ飬ÅųżĮĖĖ®”¢øÉŌļµÄ¶žŃõ»ÆĢ¼²»ÄÜŹ¹×ĻÉ«ŹÆČļ±äŗģ£¬“Ó¶ųÖ¤Ć÷ĮĖ¶žŃõ»ÆĢ¼ÓėĖ®½įŗĻÉś³ÉĖįŠŌĪļÖŹ£¬¼×Ķ¬Ń§Ōņ²»ÄÜÖ¤Ć÷
”¾½āĪö”æ
(1) Ķ¼ÖŠŅĒĘ÷¢ŚµÄĆū³Ę£ŗ׶ŠĪĘ棻
(2)ŹµŃéŹŅÖŠŅ»°ćÓĆŹÆ»ŅŹÆŗĶĻ”ŃĪĖįÖĘČ”¶žŃõ»ÆĢ¼£¬¹ŹŃ”ÓĆ¹ĢŅŗ²»¼ÓČČ×°ÖĆ£»¶žŃõ»ÆĢ¼µÄĆܶȱČæÕĘų“ó£¬ÄÜČÜÓŚĖ®ĒŅÓėĖ®·“Ó¦£¬¹ŹÖ»ÄÜÓĆĻņÉĻÅÅæÕĘų·Ø£»¹ŹŃ”ÓƵÄŅĒĘ÷ĪŖ¢Ś¢Ū¢Ü¢ß£»·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗCaCO3+2HCl=CaCl2+CO2”ü+ H2O£»
(3)¶žŃõ»ÆĢ¼²»Č¼ÉÕŅ²²»Ö§³ÖČ¼ÉÕ£¬¹ŹæÉÓĆČ¼×ŵÄľĢõ¼ģŃ飬ŃéĀśÓ¦·ÅŌŚĘææŚ£¬¹Ź¼ģŃé·½·ØĪŖ£ŗ½«Č¼×ŵÄľĢõĘ½·ÅÓŚĘææŚ£¬ČōľĢõĻØĆšŌņŅŃĀś£»
(4)¢ŁĶعż¶Ō±ČŹŌŃéæÉÖŖ£¬CO2Ź¹ŹÆČļŹŌŅŗ±äŗģÉ«µÄŌŅņŹĒ£ŗ¶žŃõ»ÆĢ¼ÓėĖ®·“Ӧɜ³ÉĮĖĢ¼Ėį£»
¢ŚŅŅĶ¬Ń§·½°øŗĻĄķ£»ŅŅĶ¬Ń§Ķعż¶Ō±ČŹµŃ飬֤Ć÷ĮĖĖ®”¢øÉŌļµÄ¶žŃõ»ÆĢ¼²»ÄÜŹ¹×ĻÉ«ŹÆČļ±äŗģ£¬“Ó¶ųÖ¤Ć÷ĮĖ¶žŃõ»ÆĢ¼ÓėĖ®½įŗĻÉś³ÉĖįŠŌĪļÖŹ£¬¼×Ķ¬Ń§Ōņ²»ÄÜÖ¤Ć÷”£


 ×ŪŗĻ×Ō²āĻµĮŠ“š°ø
×ŪŗĻ×Ō²āĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĶ¼ĪŖijĶ¬Ń§½ųŠŠµē½āĖ®ŹµŃéµÄ×°ÖĆĶ¼£¬¾Ż“Ė»Ų“š
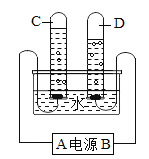
£Ø1£©ŌŚĖ®µÄµē½āŹµŃéÖŠ£¬ŌŚ±ä»ÆĒ°ŗóƻӊøıäµÄĪ¢Į£ŹĒ____________”£
£Ø2£©ÉĻŹöŹµŃéæÉŅŌµĆ³ö:Ė®ŹĒÓÉ___________________×é³ÉµÄ”£
£Ø3£©µē½āĖ®Ź±ĻČĻņĖ®²ŪÖŠ¼ÓŅ»Š©ĒāŃõ»ÆÄĘČÜŅŗ»ņĻ”ĮņĖį£¬ÄæµÄŹĒ___________”£
£Ø4£©C”¢DŹŌ¹ÜÖŠŹÕ¼Æµ½µÄĘųĢåµÄÖŹĮæ±ČŌ¼ĪŖ________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĖ®ŹĒÉśĆüÖ®Ō“£¬Ņ²ŹĒČĖĄą×ī±¦¹óµÄ׏Ō“”£ŹŌÓĆÄćѧ¹żµÄ»ÆѧÖŖŹ¶£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©½ŚŌ¼Ė®×ŹŌ“£¬·ĄÖĪĖ®ĪŪČ¾ŹĒĆæøö¹«ĆńÓ¦¾”µÄŌšČĪŗĶŅåĪń£¬ĻĀĮŠ×ö·Ø»įŌģ³ÉĖ®ĢåĪŪČ¾µÄÓŠ______£ØĢīŠņŗÅ£©
A.¹¤Ņµ·ĻĖ®Ö±½ÓÅÅ·Å B.¹¤Ņµ·ĻĘų“¦ĄķŗóÅÅ·Å
C.½ūÖ¹Ź¹ÓĆŗ¬Į×Ļ“ŅĀ·Ū D.“óĮæŹ¹ÓĆ»Æ·Ź”¢Å©Ņ©
£Ø2£©ČēĶ¼ŹĒĖ®Ķصē·Ö½āµÄŹ¾ŅāĶ¼£¬ŌŚŹµŃé¹ż³ĢÖŠ£¬ŹŌ¹ÜaÖŠ²śÉśµÄĘųĢåŹĒ________£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_____________________________________”£
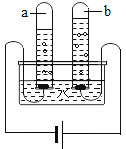
£Ø3£©×ŌČ»½ēÖŠµÄĖ®¶¼²»ŹĒ“æĖ®£¬ĄūÓĆ³Į½µ”¢Īüø½”¢_______ŗĶÕōĮóµČ·½·ØæÉŅŌ¾»»ÆĖ®”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æij°ąŃ§ÉśŌŚĄĻŹ¦µÄÖøµ¼ĻĀĢ½¾æĢśÓėĖ®ÕōĘųµÄ·“Ó¦”£
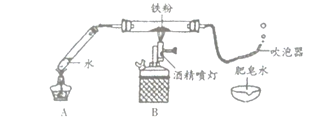
£Ø1£©°“ČēĶ¼ĖłŹ¾×°ŗĆŅ©Ę·”¢Į¬ŗĆ×°ÖĆ£Ø¼Š³ÖŅĒĘ÷ŅŃĀŌČ„£©”£ĘäÖŠA×°ÖƵÄ×÷ÓĆŹĒ______”£
£Ø2£©¼ÓČČŅ»¶ĪŹ±¼äŗó£¬B×°ÖĆÖŠµÄ»ŅÉ«Ģś·ŪÖš½„±äŗŚ£¬“µÅŻĘ÷Į¬Šų“µ³öĘųÅŻ£¬ĒŅĘųÅŻĻņÉĻ·ÉĘš£»ÓĆČ¼×ŵÄľĢõææ½üĘųÅŻ£¬ÄܲśÉś±¬ĆłÉł”£øĆĘųĢåŹĒ______”£
£Ø3£©Ķ¬Ń§ĆĒĢÖĀŪŗóČĻĪŖ£ŗĢśÓėĖ®ÕōĘų·“Ӧɜ³ÉµÄ¹ĢĢåŹĒ”°ĢśµÄŅ»ÖÖŃõ»ÆĪļ”±”£²£Į§¹ÜÄŚµÄŗŚÉ«¹ĢĢåÖŠ»¹æÉÄÜŗ¬ÓŠ”°Ź£ÓąµÄĢś”±”£
£Ø²éŌÄ׏ĮĻ£©ĢśµÄŃõ»ÆĪļÓŠČżÖÖ£ŗ![]() £¬ĘäÖŠ
£¬ĘäÖŠ![]() ŹĒŗģ×ŲÉ«µÄ£¬¶ų
ŹĒŗģ×ŲÉ«µÄ£¬¶ų![]() ½Ó“„µ½æÕĘų»įÓÉŗŚÉ«±äĪŖŗģ×ŲÉ«”£
½Ó“„µ½æÕĘų»įÓÉŗŚÉ«±äĪŖŗģ×ŲÉ«”£
£ØŹµŃéĢ½¾æ£©
¢Ł½«¹ÜÖŠµÄŗŚÉ«¹ĢĢåµ¹³ö£¬Ę½ĘĢÓŚ°×Ö½ÉĻ£¬Ć»ÓŠ·¢ĻÖŗģ×ŲÉ«ĪļÖŹ£¬ĖµĆ÷Éś³ÉµÄ¹ĢĢå²»æÉÄÜŹĒ______£¬Ņ»»į¶łÖ®ŗó£¬ŗŚÉ«¹ĢĢå²»±äÉ«£¬ŌņŗŚÉ«¹ĢĢåÖŠŅ»¶Øƻӊ______”££ØĢīĪļÖŹĆū³Ę£©
¢ŚČ”ÉĻŹöŗŚÉ«¹ĢĢåÉŁŠķ¼ÓČė×ćĮæµÄ![]() ČÜŅŗ£¬·¢ĻÖŗŚÉ«¹ĢĢå²æ·ÖČܽā£¬ĒŅÓŠ______É«¹ĢĢåĪļÖŹ³öĻÖ£¬ĖµĆ÷ŗŚÉ«¹ĢĢåÖŠŅ»¶ØÓŠĢśŗĶĖÄŃõ»ÆČżĢś”£
ČÜŅŗ£¬·¢ĻÖŗŚÉ«¹ĢĢå²æ·ÖČܽā£¬ĒŅÓŠ______É«¹ĢĢåĪļÖŹ³öĻÖ£¬ĖµĆ÷ŗŚÉ«¹ĢĢåÖŠŅ»¶ØÓŠĢśŗĶĖÄŃõ»ÆČżĢś”£
£ØĢ½¾æ½įĀŪ£©ĢśÓėĖ®ÕōĘų·¢ÉśÖĆ»»·“Ó¦£¬Éś³ÉµÄŗŚÉ«¹ĢĢåŹĒ![]() £¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_______”£
£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æij°ąŃ§ÉśŌŚĄĻŹ¦µÄÖøµ¼ĻĀĢ½¾æĢśÓėĖ®ÕōĘųµÄ·“Ó¦”£
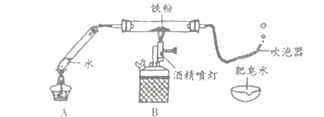
£Ø1£©°“ČēĶ¼ĖłŹ¾×°ŗĆŅ©Ę·”¢Į¬ŗĆ×°ÖĆ£Ø¼Š³ÖŅĒĘ÷ŅŃĀŌČ„£©”£ĘäÖŠA×°ÖƵÄ×÷ÓĆŹĒ______”£
£Ø2£©¼ÓČČŅ»¶ĪŹ±¼äŗó£¬B×°ÖĆÖŠµÄ»ŅÉ«Ģś·ŪÖš½„±äŗŚ£¬“µÅŻĘ÷Į¬Šų“µ³öĘųÅŻ£¬ĒŅĘųÅŻĻņÉĻ·ÉĘš£»ÓĆČ¼×ŵÄľĢõææ½üĘųÅŻ£¬ÄܲśÉś±¬ĆłÉł”£øĆĘųĢåŹĒ______”£
£Ø3£©Ķ¬Ń§ĆĒĢÖĀŪŗóČĻĪŖ£ŗĢśÓėĖ®ÕōĘų·“Ӧɜ³ÉµÄ¹ĢĢåŹĒ”°ĢśµÄŅ»ÖÖŃõ»ÆĪļ”±”£²£Į§¹ÜÄŚµÄŗŚÉ«¹ĢĢåÖŠ»¹æÉÄÜŗ¬ÓŠ”°Ź£ÓąµÄĢś”±”£
£Ø²éŌÄ׏ĮĻ£©ĢśµÄŃõ»ÆĪļÓŠČżÖÖ£ŗ![]() £¬ĘäÖŠ
£¬ĘäÖŠ![]() ŹĒŗģ×ŲÉ«µÄ£¬¶ų
ŹĒŗģ×ŲÉ«µÄ£¬¶ų![]() ½Ó“„µ½æÕĘų»įÓÉŗŚÉ«±äĪŖŗģ×ŲÉ«”£
½Ó“„µ½æÕĘų»įÓÉŗŚÉ«±äĪŖŗģ×ŲÉ«”£
£ØŹµŃéĢ½¾æ£©
¢Ł½«¹ÜÖŠµÄŗŚÉ«¹ĢĢåµ¹³ö£¬Ę½ĘĢÓŚ°×Ö½ÉĻ£¬Ć»ÓŠ·¢ĻÖŗģ×ŲÉ«ĪļÖŹ£¬ĖµĆ÷Éś³ÉµÄ¹ĢĢå²»æÉÄÜŹĒ______£¬Ņ»»į¶łÖ®ŗó£¬ŗŚÉ«¹ĢĢå²»±äÉ«£¬ŌņŗŚÉ«¹ĢĢåÖŠŅ»¶Øƻӊ______”££ØĢīĪļÖŹĆū³Ę£©
¢ŚČ”ÉĻŹöŗŚÉ«¹ĢĢåÉŁŠķ¼ÓČė×ćĮæµÄ![]() ČÜŅŗ£¬·¢ĻÖŗŚÉ«¹ĢĢå²æ·ÖČܽā£¬ĒŅÓŠ______É«¹ĢĢåĪļÖŹ³öĻÖ£¬ĖµĆ÷ŗŚÉ«¹ĢĢåÖŠŅ»¶ØÓŠĢśŗĶĖÄŃõ»ÆČżĢś”£
ČÜŅŗ£¬·¢ĻÖŗŚÉ«¹ĢĢå²æ·ÖČܽā£¬ĒŅÓŠ______É«¹ĢĢåĪļÖŹ³öĻÖ£¬ĖµĆ÷ŗŚÉ«¹ĢĢåÖŠŅ»¶ØÓŠĢśŗĶĖÄŃõ»ÆČżĢś”£
£ØĢ½¾æ½įĀŪ£©ĢśÓėĖ®ÕōĘų·¢ÉśÖĆ»»·“Ó¦£¬Éś³ÉµÄŗŚÉ«¹ĢĢåŹĒ![]() £¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_______”£
£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æA-FŗĶX¶¼ŹĒ³õÖŠ»Æѧ֊µÄ³£¼ūĪļÖŹ£¬ĘäÖŠA”¢CŹĒŌŖĖŲ×é³ÉĻąĶ¬µÄĮ½ÖÖĘųĢ壬B”¢FŹĒŗģÉ«¹ĢĢ壬ĖüĆĒµÄ×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾£Ø²æ·ÖÉś³ÉĪļŅŃŹ”ĀŌ£©£ŗ

£Ø1£©ĒėŠ“³öFµÄ»ÆѧŹ½_____________________£»
£Ø2£©Š“³ö·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½___________________£»
£Ø3£©Š“³ö¢ŚµÄ»Æѧ·½³ĢŹ½___________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĄūÓĆŗ£Ė®ĢįČ”ŃĪµÄ¹ż³ĢČēĶ¼ĖłŹ¾”£»Ų“šÓŠ¹ŲĪŹĢā£ŗ

(1)Ņ»¶ØÖŹĮæµÄŗ£Ė®£¬ĶعżÖüĖ®³ŲŅżČėµ½Õō·¢³ŲÖŠ£¬ŌŚĆ»ÓŠŅżČė½į¾§³ŲÖ®Ē°µÄÕō·¢·¢¹ż³ĢÖŠ£¬Õō·¢³ŲÖŠĒā»ÆÄʵÄÖŹĮæ»į______(Ģī”°Ōö“ó”±”¢”°²»±ä”±»ņ”°¼õŠ””±)”£ŌŚÕō·¢³ŲÖŠ“Ł½ųĖ®·ÖÕō·¢£¬Ö÷ŅŖŹĒĄūÓĆĮĖĻĀĮŠø÷ĻīÖŠµÄ______(ĢīŃ”ĻīŠņŗÅ)”£
¢Ł³±Ļ«ÄÜ¢įÉśĪļÄÜ¢ŪµēÄÜ¢ÜĢ«ŃōÄÜ¢Ż»ÆѧÄÜ
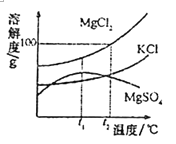
(2)ɹŃĪ¹ż³ĢÖŠµĆµ½“ÖŃĪŗĶÄøŅŗ”£ÄøŅŗµÄÖ÷ŅŖ³É·Ö¼°ĘäČܽā¶ČµÄ±ä»ÆČēĶ¼£¬t2”ꏱMgCl2µÄČܽā¶ČĪŖ______£¬t2”ꏱ£¬½«µČÖŹĮæµÄMgCl2”¢KClŗĶMgSO4ČżÖÖĪļÖŹµÄ±„ŗĶČÜŅŗ·Ö±š½µĪĀÖĮt1”ꏱ£¬ĪŽ¾§ĢåĪö³öµÄŹĒ______(Ģī»ÆѧŹ½)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĖ®ŹĒ±¦¹óµÄ×ŌȻ׏ĮĻ£¬ČĆĪŅĆĒŅ»Ęš×ß½ų”°Ė®”±µÄŹĄ½ē”£
¢ŁĻĀĶ¼µē½āĖ®ŹµŃéÖŠ£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_______”£Ė®µē½ā¹ż³Ģ·¢ÉśøıäµÄĪ¢Į£Ćū³Ę_______”£
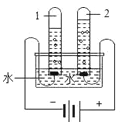
¢ŚŅ½ĮĘÉĻ³£ÓĆ0.9£„![]() ČÜŅŗ×÷ÉśĄķŃĪĖ®£¬ÅäÖĘ1000gÉśĄķŃĪĖ®ŠčŅŖĀČ»ÆÄĘ¹ĢĢåµÄÖŹĮæĪŖ_______”£Ė®»¹æÉŅŌÖĘČ”Ģ¼ĖįŅūĮĻ£¬øĆ¹ż³ĢÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_______”£
ČÜŅŗ×÷ÉśĄķŃĪĖ®£¬ÅäÖĘ1000gÉśĄķŃĪĖ®ŠčŅŖĀČ»ÆÄĘ¹ĢĢåµÄÖŹĮæĪŖ_______”£Ė®»¹æÉŅŌÖĘČ”Ģ¼ĖįŅūĮĻ£¬øĆ¹ż³ĢÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŹµŃéæĪÉĻ£¬ĄīĄĻŹ¦½«Ź§Č„±źĒ©µÄŅ»ÖÖ¹ĢĢåAŗĶŅ»ÖÖŅŗĢåB»ģŗĻŗ󣬷¢ĻÖÓŠĘųÅŻĆ°³ö”£Ģį³öĪŹĢā£ŗ²śÉśµÄŹĒÄÄÖÖĘųĢå£æ
²ĀĻėŅ»£ŗ¶žŃõ»ÆĢ¼”£
²ĀĻė¶ž£ŗŃõĘų£¬ŅĄ¾ŻŹĒ_______£ØÓĆ»Æѧ·½³ĢŹ½±ķŹ¾£©
²ĀĻėČż_______£¬ŅĄ¾ŻŹĒ_______£ØÓĆ»Æѧ·½³ĢŹ½±ķŹ¾£©
ŹµŃé²½Öč | ŹµŃéĻÖĻóÓė»Æѧ·½³ĢŹ½ | ŹµŃé½įĀŪ |
·½°øŅ»£ŗ·Ö±šČ”Į½ÖÖŅ©Ę·ÉŁŠķÓŚŅ»Ö§ŹŌ¹ÜÖŠ£¬²¢½«Č¼×ŵÄľĢõÉģČėŹŌ¹ÜÖŠ”£ | ĻÖĻó£ŗ_______”£ | ²ĀĻėŅ»ÕżČ· |
·½°ø¶ž£ŗ_______”£ | ĻÖĻó£ŗ_______£¬»Æѧ·½³ĢŹ½£ŗ_______”£ |
ŹµŃé·“Ė¼£ŗ¹ĢĢåAŗĶŅŗĢåB»ģŗĻŗóæÉÄÜ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_______”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com