
 ���Ʋ�ϡ��һ������������Na2SO4��Һ��
���Ʋ�ϡ��һ������������Na2SO4��Һ������ ��1���ڸ��ݳ���3gҩƷ�IJ��������ش�
�۸�������Ͳ��ȡ47.0mL�IJ�������ͼʾ��
��2�����������������估��Һ���ܶȡ��������֮��Ĺ�ϵ�������㣮
���  �⣺��1������������ƽ����3.0g��Na2SO4����ƽ����ֱ�����ƽ�������̷���������ͬ��ֽƬ���Ȱ��������3gλ�ã�Ȼ��������������ҩƷ����������ƽǡ��ƽ�⣮
�⣺��1������������ƽ����3.0g��Na2SO4����ƽ����ֱ�����ƽ�������̷���������ͬ��ֽƬ���Ȱ��������3gλ�ã�Ȼ��������������ҩƷ����������ƽǡ��ƽ�⣮
����ȡ������Ͳ��ȡ47.0mLˮ��47.0mLˮ��Һ��λ����ͼ��
��2������Һa�����������ǣ�$\frac{1mL��1g/mL��6%}{100mL��1g/mL}��100%$=0.06%
��������Һ������Ϊ��$\frac{3g}{0.06%}$=5000g������ǣ�$\frac{5000g}{1g/mL}$=5000mL
�ʴ�Ϊ����1��������������3g���룬������������ҩƷ���ۼ���ͼ����2��5000��
���� ������Ҫ����������һ������������Һ�Ļ����������ѶȲ����ڲ�����һ��Ҫץס������Ҫ�㣬ȷ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
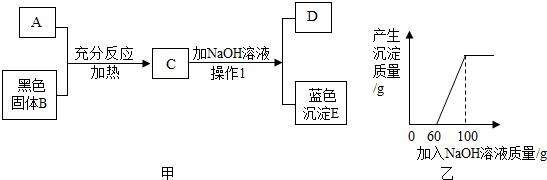
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ο���ͼ��Ϣ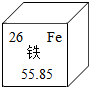 ���������ԭ������Ϊ26 ���������ԭ������Ϊ26 | |
| B�� | �������dz��������Ͻ� | |
| C�� | �����ڳ�ʪ�Ŀ������������⣬��˸�����ʹ��Ҫע����� | |
| D�� | �ó������¯�����Ļ�ѧ��Ӧ����ʽ��Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
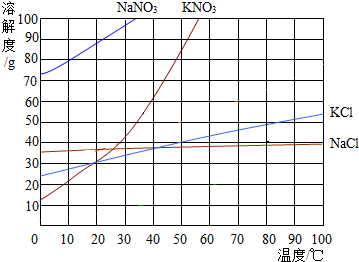 ��ͼΪ���ֹ�����ܽ�����ߣ��ش��������⣺
��ͼΪ���ֹ�����ܽ�����ߣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ��ԭ�Ӻ���������Ϊ6 | B�� | ��Ԫ��λ��Ԫ�����ڱ��е������� | ||
| C�� | �����ӣ�Al3+���������������Ӳ� | D�� | ��ԭ�ӵ�����Ϊ55.85g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
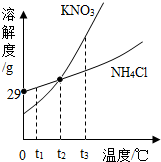 KNO3��NH4Cl���ܽ��������ͼ��ʾ������˵��������ǣ�������
KNO3��NH4Cl���ܽ��������ͼ��ʾ������˵��������ǣ�������| A�� | �����£�NH4Cl������ˮ | |
| B�� | t2�棬������Һ�����ʵ���������һ����� | |
| C�� | t3�棬KNO3���ܽ�ȴ���NH4Cl���ܽ�� | |
| D�� | ���ֱ�����Һ��t2�潵��t1��ʱ�����о������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com