
���� ��1�����ݻ���̿�������Է�����
��2��������Һ��������ϴ�ྫ���黯���÷�����
��3�����ݷ����ڲ����˶�������������
��4����������غ��е�ũ������Ҫ��Ӫ��Ԫ�ص����������
��5����������ԭ�������ش�
��6�����������ᣨC6H8O7���Ļ�ѧʽ���㣮
��7�����ݰ�����N2O��Ӧд����ѧ����ʽ��
��� �⣺��1�����ڻ���̿���������ԣ����þ�ˮ���к��д�������̿����������������
��2����ʳ�Ρ������ͷֱ����ˮ�У����γ���Һ����ʳ�Σ��ٷֱ����ϴ�ྫ���ܳ����黯������� �����ͣ�
��3���ڳ�������ʱ���ڿ��������ŵ�����ζ����Ҫԭ���Ƿ����ڲ����˶����������ͨ���˶���ɢ����Χ�Ŀ����У�
��4����ũҵ�����У�ʩ�õ�����أ�KNO3������ũ������������Ҫ�ļء�������Ԫ�أ����ڸ��Ϸ��ϣ�
��5����ɭ�ַ�������ʱ���Ȼ�Ĵ�ʩ֮һ�ǽ����������·ǰ��һƬ��ľ�������γɸ��������ԭ���������ȼ�
��6���������ᣨC6H8O7���У�̼����Ԫ�ص�������Ϊ��12��6������1��8��=9��1��
��7���ڴ����������£�����ʹ����β���еĵ����������ת�������ĵ�����ˮ��������Ӧ�Ļ�ѧ����ʽ�ǣ�2NH3+3N2O$\frac{\underline{\;����\;}}{\;}$4N2+3H2O��
�ʴ�Ϊ��1����������2��A�� B ��3�������ڲ����˶�����4�����ϣ���5�������ȼ���6��9��1��
��7��2NH3+3N2O$\frac{\underline{\;����\;}}{\;}$4N2+3H2O��
���� ������ѶȲ����������֪ʶ�����ڿα��Ļ���֪ʶ��������ϵ������ܣ�ѧ�����û�ѧ��֪ʶ����������е�һЩ���⣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ձ����Թܡ������� | B�� | �ձ�����ͷ�ιܡ������� | ||
| C�� | �ձ�����ͷ�ιܡ�©�� | D�� | �ձ����ƾ��ơ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �����жԸ����ӵ�˵���в���ȷ���ǣ�������
�����жԸ����ӵ�˵���в���ȷ���ǣ�������| A�� | ��������Ƿֲ��Ų��� | |
| B�� | ����һ����10������ | |
| C�� | �����ȶ��ṹ | |
| D�� | �����������ӻ������ӻ�ϡ������ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
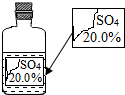 һ��Ȥζ��ѧ��У�����ʦ��ͬѧ��չʾ��һƿ��ǩ�������ɫ��Һ������ͼ��ʾ��Ҫ��ͬѧ�ǽ���̽����ȷ����ƿ��Һ������ʲô��Һ��
һ��Ȥζ��ѧ��У�����ʦ��ͬѧ��չʾ��һƿ��ǩ�������ɫ��Һ������ͼ��ʾ��Ҫ��ͬѧ�ǽ���̽����ȷ����ƿ��Һ������ʲô��Һ��| ���� | MgSO4 | Na2SO4 | ��NH4��2SO4 | H2SO4 |
| �ܽ�� | 35.1g | 19.5g | 75.4g | ��ˮ����Ȼ��� |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| aȡ����Һ�������Թ��У������еμӼ�������������Һ | ��Һ���а�ɫ�������� | ����ٳ��� |
| bȡ����Һ�������Թ��У������еμӼ�����ɫʯ����Һ | ��Һ���ɫ | ����۳��� |
| ʵ����� | ʵ������ | ʵ����� |
| ȡ����Һ�������Թ��У���������Һ����NaOH�������𰸺���Ҳ�ɣ���Һ�����ȣ� | �����д̼���ζ������ | ����ܳ������÷�Ӧ�Ļ�ѧ����ʽΪ��NH4��2SO4+2NaOH=Na2SO4+2H2O+2NH3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
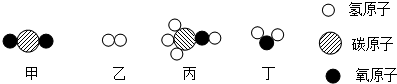
| A�� | ������Ӧ�ļ��ҵ�������Ϊ 22��3 | |
| B�� | ������Է�������Ϊ 24 | |
| C�� | ��Ӧǰ������������� | |
| D�� | ���ɱ��Ͷ��ķ��Ӹ�����Ϊ 2��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH4+��Na+��SO42- | B�� | H+��K+��CO32- | C�� | Ba2+��OH-��H+ | D�� | Cu2+��NO3-��OH- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com