
(6��)ij��ȤС���ͭ����ȡ����ɫ��ĩ���������ʵ�鲽�����̽��ͭ�̵���ɡ�

��д����B���ʵĻ�ѧʽ ��ͭ�̵����Ԫ�� ��
���ҹ��Ŵ��С����������Ϊͭ���ļ��أ������ִ�ʪ��ұ���ԭ������������ʵ�鷽���е� ���裨�����ֱ�ţ����ƣ���ѧ����ʽΪ ��
��д���ڢ۲���Ӧ�Ļ�ѧ����ʽ�� ��
������Ʒ������Тݢޢ�����ұ��ͭ�ķ�����ͨ���Ƚ������Դ����Դ���ȷ��棬˵������һ�ַ��������е��ŵ㣺 ��
(1)CuO��Cu��H��O��C��
(2)�ݣ�Fe+CuSO4=FeSO4+Cu
(3) 2H2O  2H2��+ O2��
2H2��+ O2��
(4)����������ͭ��Ӧ�ķ���ұ��ͭ�������������(���������𰸿ɸ���)
��������
�����������1�����������֪����ɫ��ĩB��ϡ���ᷴӦ������ɫ��ҺE����E��һ������ͭ���ӣ����ɫ��ĩBΪ����ͭ����ɫ��ҺEΪ����ͭ��Һ������ͭ��Һ�ܺ��������û���Ӧ������ͭ����ɫ���壩����������������HΪ��ɫ���壬��HΪͭ����Ϊ��ɫҺ��C��ͨ���������ֽܷ⣬������ѧ��ѧ֪ʶ��֪��CΪˮ������ˮ���������������������FΪ�����������е�һ�֣��ָ���ͼʾ��F������ͭ����������ͭ��˵��F���л�ԭ�ԣ���F������������ͼʾ��GҲ��������ͭ���ȷ�Ӧ������ͭ��˵��GҲ���л�ԭ�ԣ���G����D�����ľ̿���·�Ӧ���ɵģ���GΪһ����̼����DΪ������̼������ͼʾ��֤����ȷ�����ݻ�ѧ��Ӧǰ��Ԫ�ص�������֪��ͭ�̵����Ԫ��ΪCu��O��C��H��
��2�������������Ϊͭ����ָ��������ͭ��Һ������Ӧ������ͭ�������������䷴Ӧ�Ļ�ѧ����ʽ��Fe+CuSO4=FeSO4+Cu������ͼʾ�еĵڢݲ����ơ�
��3���ڢ۲��ķ�Ӧ��ˮ�ĵ�⣬�������������������䷴Ӧ�Ļ�ѧ����ʽ��2H2O 2H2��+O2����
2H2��+O2����
��4��ʵ�鷽�����۵�һ��ԭ���ǣ�(1)������ȷ��(2)�������У�(3)���ú�����(4)�������ܣ�(5)������������һ�㲻���ȣ�������㣬��Ⱦ�٣���Լ��Դ�ķ�����Ϊ�Ϻõķ�����
���㣺ʵ��̽�����ʵ���ɳɷ��Լ����������ʵ�Ԫ����ɣ���ѧʽ����д�����壬����������ұ����������Ӧ����ͱ��ʵ���ϵ����д��ѧ����ʽ
�����������Ĺؼ��Ǹ������������ҵ�ͻ�Ƶ㣬����Ҫ�������ۡ��������������Եģ�Ҫ����ѧ����֪ʶ�Ƚ���������ԵIJ��룬Ȼ�����Ų�������Ƶ���ֻҪ���������㣬����ͳ������粻���㣬���ټ��裬����֤��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��6�֣���ѧ��ȤС���ͬѧȡ10 gijп��Ʒ�����������ʣ������ʲ�����ˮ��Ҳ�����ᷴӦ�����ձ��У������м���һ������ϡ���ᣬ������ϡ���������Ϊ93��7 gʱ��ǡ����ȫ��Ӧ����������������뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ���Իش��������⣺

��1����ͼ�п��Կ�������ȫ��Ӧ����������������Ϊ g��
��2����Ʒ��п������Ϊ���٣���3����Ӧ��������Һ������п����������Ϊ���٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
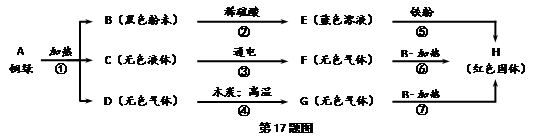
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ̩���а�����ѧ���꼶��ѧ�ڵ�һ���¶ȼ�⻯ѧ�Ծ����������� ���ͣ��ƶ���
(6��)ij��ȤС���ͭ����ȡ����ɫ��ĩ���������ʵ�鲽�����̽��ͭ�̵���ɡ�
��д����B���ʵĻ�ѧʽ ��ͭ�̵����Ԫ�� ��
���ҹ��Ŵ��С����������Ϊͭ���ļ��أ������ִ�ʪ��ұ���ԭ������������ʵ�鷽���е� ���裨�����ֱ�ţ����ƣ���ѧ����ʽΪ ��
��д���ڢ۲���Ӧ�Ļ�ѧ����ʽ�� ��
������Ʒ������Тݢޢ�����ұ��ͭ�ķ�����ͨ���Ƚ������Դ����Դ���ȷ��棬˵������һ�ַ��������е��ŵ㣺 ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�ij��и��ƾ��꼶��һѧ����ĩѧҵ���Ի�ѧ�Ծ� ���ͣ�������
��6�֣���ѧ��ȤС���ͬѧȡ10 gijп��Ʒ�����������ʣ������ʲ�����ˮ��Ҳ�����ᷴӦ�����ձ��У������м���һ������ϡ���ᣬ������ϡ���������Ϊ93��7 gʱ��ǡ����ȫ��Ӧ����������������뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ���Իش��������⣺

��1����ͼ�п��Կ�������ȫ��Ӧ����������������Ϊ g��
��2����Ʒ��п������Ϊ���٣���3����Ӧ��������Һ������п����������Ϊ���٣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com