
��9�֣���Ϊ�ⶨij��ʯ��ʯ��̼��Ƶ�������������ȤС��ͬѧȡһ��������ʯ��ʯ���ձ��У����ձ��������Ũ�����ᣬ��Ӧ���̲��ʣ�������������������������ϵ��ͼ��ʾ��ʯ��ʯ�е����ʲ������ᷴӦ��Ҳ������ˮ������ش��������⣺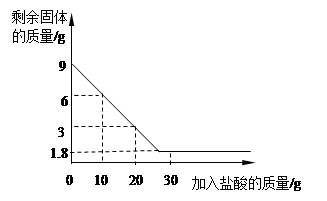
��1��ʯ��ʯ���������ʵ������� g��
��2��ͨ�������������������������������
��3����ȤС��ͬѧ�òⶨ�������������������ʯ��ʯ��̼��Ƶ������������������ȷ��ͨ������õ���̼��Ƶ�����������ʵ����ֵƫ����ԭ������ǣ� ��
��1��1.8 ��2��21.9%��3��������̼�����к��н�Ũ����ӷ����Ȼ�������ʹ��������ʵ�ʲ���������̼�������ɶ�����̼�����̼�������ƫ��
�������������ʯ��ʯ�е����ʲ������ᷴӦ��Ҳ������ˮ�����Թ�����ٵ�������Ϊ̼��Ƶ�������9-1.8=7.2g�����ʵ�������������ʣ�������Ϊ1.8g����2������������������ʱ��Ҫע�⣬Ӧ���÷�Ӧ����������������㣬���Կ�ѡ��10g����20g����10g����������HCl������Ϊx (Ҳ����20g������㣬������������Ҳ�÷�)CaCO3+2HCl=CaCl2+H2O+CO2��
100 73
9g-6g X 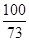 =
=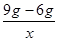
x=2.19g
���������ʵ���������=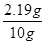 ��100%=21.9%
��100%=21.9%
��3��������̼�����к��н�Ũ����ӷ����Ȼ�������ʹ��������ʵ�ʲ���������̼�������ɶ�����̼�����̼�������ƫ��
���㣺���ݻ�ѧ����ʽ���м���


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ʯ��ʯ���Ƶ������ƣ�����ʯ��ʯ�е����ʲ����뷴Ӧ���Ҳ����ơ�̼Ԫ�أ���������պ�ʣ������и�Ԫ����̼Ԫ�ص�������Ϊ20��3�����ѷֽ��̼���ռԭ̼��Ƶ���������Ϊ�� ��
| A��40% | B��60% | C��30% | D��50% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ijͬѧ�ҵ���ˮ�ܵ������ˣ�����������һƿ���ܵ���ͨ����(����˵����ͼ)����ͨ����������������������Һ��Ӧʱ�ų��������ȣ��Լӿ�����������ë�����ٻ�������ã���Ӧ��ԭ��Ϊ��2Al+2NaOH+2H20 2NaAl02+3H2������ش������й����⣺
��1���ùܵ���ͨ����Ҫ�ܷⱣ���ԭ���� ��
��2������ʹ��˵������ͬѧ�ԡ���ͨ�������˽�һ���о���
�����ձ��м���206gˮ���ټ��뱾Ʒ200g��������
ȫ����Ӧ����(����������1λС��)
�����������������(������һ0.09g��L)
�ڷ�Ӧ����Һ���������Ƶ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
(7��)ij��ȤС����ʵ���ҷ���һƿ���ھ��õ��������ƺ�һƿδ��Ũ�ȵ�ϡ���ᡣΪ�ⶨ�������Ƶı��������ϡ�����Ũ�ȣ�����ȡ9.3g���ʵ�����������Ʒ����ƿ�У�����50gˮ������ܽ⣬������ƿ�еμ�δ֪Ũ�ȵ�ϡ���ᡣʵ���ü���ϡ�������������ƿ�����ʵ�������ϵ����ͼ��ʾ��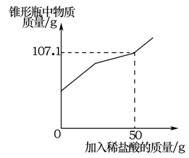
��1��9.3g��Ʒ��̼���Ƶ�������
��2��δ֪Ũ�ȵ�ϡ�������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��7�֣�ѧ��������Ժ���˼ͬѧͨ��ʵ��̽��п����������������������������Һ�ķ�Ӧ��ʵ��������������£�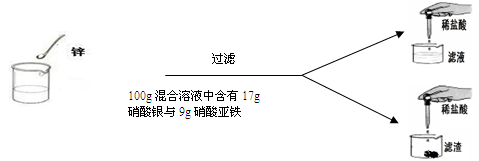
| | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| �����������������Ļ����Һ | 100g | 100g | 100g | 100g |
| � | 2g | 3.25g | m | 9.75g |
| ����Һ�м���ϡ������ʵ������ | ������ɫ���� | ���������� | ���������� | ���������� |
| �������м���100 gϡ������ʵ������ | ���������� | ���������� | �������ݣ���Һ��Ϊdz��ɫ | �������������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
(5��) 55.7 g̼������Һǡ����36.5 gij������Һ��ȫ��Ӧ����÷�Ӧ����Һ������Ϊ90 g����
��1�������˶��ٿ˶�����̼��
��2����Ӧ��������Һ���������������Ƕ��٣�
��3������ϡ���������ʵ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��3�֣�ij��ѧ��ȤС���ͬѧ��һ�ݹ�����Ʒ������̽����ͨ��ʵ����ȷ������Ʒ�������������ۻ�϶��ɡ����dz�ȡ��13.6g������Ʒ����ͼ1��ʾ��װ�ü���ʵ�飬�ⶨ�IJ���������ͼ2��ʾ��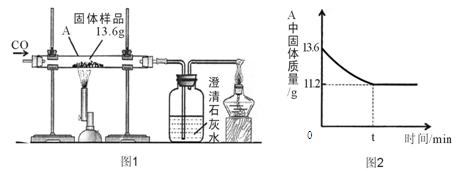
����㣺������Ʒ���������������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��3�֣��綯���г���С�����Ƚ�ͨ�����ж���Ϊ���ṩ���ܵ�Ǧ���أ��ֳơ���ƿ�����������ŵ��ǿ��Գ��ѭ��ʹ�ã���ƿ�ڷ����Ļ�ѧ��Ӧ�ǣ�
PbO2���̣�+ 2H2SO4 + Pb = 2PbSO4��+ 2H2O
ij��ƿ��װ��36%��ϡ����1200 g����ƿ����ʱ��310��5 g��Ǧ�μӷ�Ӧ���Լ��㣺
��1��ԭϡ�������������ʵ������� g��
��2����ƿ����ʱ���������������
��3����Ӧ��������Һ�����ʵ��������������������ȷ��0��01%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
����β��ϵͳ��ʹ�ô�ת�������ɽ���CO��NO���ж�������ŷţ��䷴Ӧ��ѧ����ʽΪ��2CO+2NO����2CO2+N2������5��6gCO��ת��ʱ������ͬʱ��ת����NO��������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com