
СϣΪ�˲ⶨ���������������CO2������������������ʵ�飺���ռ�5 L������������Ʒ������������Ʒ�м���������NaOH��Һ����ַ�Ӧ���۰ѷ�Ӧ�����Һת�Ƶ��ձ��У���������5�������ᣬ������������Ϊֹ����������������������������Ĺ�ϵ����ͼ��(CO2���ܶ�Ϊ1.96g/L�����������λС��)
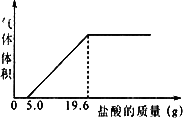
(1)����5.0 g����ǰû�����ݲ�����ԭ����________(�û�ѧ����ʽ��ʾ)��
(2)�����������CO2���������Ƕ��٣�
(3)��������Ʒ��CO2���������Ϊ________����������Ŀ�����CO2���������________�����Դ���Ľ�����________��
 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
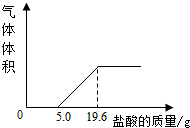 СϣΪ�˲ⶨ���������������CO2������������������ʵ�飺���ռ�5L������������Ʒ������������Ʒ�м��������NaOH��Һ����ַ�Ӧ���۰ѷ�Ӧ�����Һת�Ƶ��ձ��У���������5%�����ᣬ������������Ϊֹ����������������������������Ĺ�ϵ��ͼ���� CO2���ܶ�Ϊ1.96g/L�����������λС������1������5.0g����ǰû�����ݲ�����ԭ����
СϣΪ�˲ⶨ���������������CO2������������������ʵ�飺���ռ�5L������������Ʒ������������Ʒ�м��������NaOH��Һ����ַ�Ӧ���۰ѷ�Ӧ�����Һת�Ƶ��ձ��У���������5%�����ᣬ������������Ϊֹ����������������������������Ĺ�ϵ��ͼ���� CO2���ܶ�Ϊ1.96g/L�����������λС������1������5.0g����ǰû�����ݲ�����ԭ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 СϣΪ�˲ⶨ���������������CO2������������������ʵ�飺���ռ�5L������������Ʒ������������Ʒ�м��������NaOH��Һ����ַ�Ӧ���۰ѷ�Ӧ�����Һת�Ƶ��ձ��У���������5%�����ᣬ������������Ϊֹ����������������������������Ĺ�ϵ��ͼ��
СϣΪ�˲ⶨ���������������CO2������������������ʵ�飺���ռ�5L������������Ʒ������������Ʒ�м��������NaOH��Һ����ַ�Ӧ���۰ѷ�Ӧ�����Һת�Ƶ��ձ��У���������5%�����ᣬ������������Ϊֹ����������������������������Ĺ�ϵ��ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 СϣΪ�˲ⶨ���������������CO2������������������ʵ�飺���ռ�5L������������Ʒ������������Ʒ�м��������NaOH��Һ����ַ�Ӧ���۰ѷ�Ӧ�����Һת�Ƶ��ձ��У���������5%�����ᣬ������������Ϊֹ����������������������������Ĺ�ϵ��ͼ���� CO2���ܶ�Ϊ1.96g/L�����������λС����
СϣΪ�˲ⶨ���������������CO2������������������ʵ�飺���ռ�5L������������Ʒ������������Ʒ�м��������NaOH��Һ����ַ�Ӧ���۰ѷ�Ӧ�����Һת�Ƶ��ձ��У���������5%�����ᣬ������������Ϊֹ����������������������������Ĺ�ϵ��ͼ���� CO2���ܶ�Ϊ1.96g/L�����������λС�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������һ���п���ѧһģ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�걱�����������п���ѧ��ģ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com