
��5�֣�MnO2��һ����Ҫ�������ܲ��ϣ���MnO2�����н϶��MnO��MnCO3�����ᴿ�ǹ�ҵ��������Ҫ���ڡ���ͼ��ij�о���ѧϰС����ʵ������ģ�ҵ�ϴ�MnO2ת��Ϊ��MnO2�Ĺ������̡�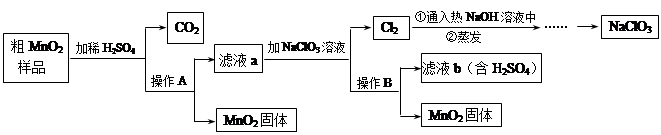
��ش��������⣺
��1������A�������� ��
��2��NaClO3����Ԫ�صĻ��ϼ�Ϊ �ۡ�
��3���������������������̨������Ȧ���������ƾ��ƺ� ��
��4��д����ϡH2SO4ʱ����CO2�Ļ�ѧ����ʽ ��
��5���������п���ѭ�����õ�������H2SO4�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�츣��ʡ�����Ӱ���ѧ���꼶�п�ģ�⻯ѧ�Ծ����������� ���ͣ��ʴ���
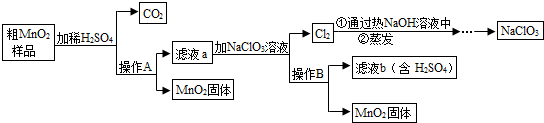
MnO2��һ����Ҫ�������ܲ��ϣ���Mn02(���н϶��Mn0��MnC03)���ᴿ�ǹ�ҵ��������Ҫ���ڡ���ͼ��ij�о���ѧϰС����ʵ������ģ�ҵ�ϴ�Mn02ת��Ϊ��Mn02�Ĺ������̡�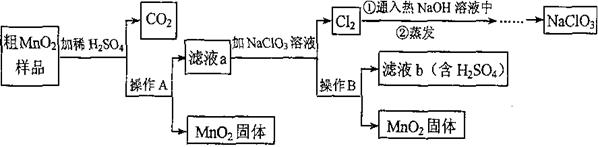 ��ش��������⣺
��ش��������⣺
(1)NaCl03����Ԫ�صĻ��ϼ�Ϊ �ۡ�
(2)����A�������� ��
(3)�������������������̨(����Ȧ)�����������ƾ��ƺ� �����в����������� ��
(4)д����MnO2��Ʒ��ϡH2SO4ʱ����C02�Ļ�ѧ����ʽ ��
(5)�������п���ѭ�����õ�������H2SO4�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ���꼶�п�ģ�⻯ѧ�Ծ��������棩 ���ͣ������
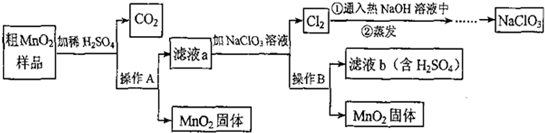
MnO2��һ����Ҫ�������ܲ��ϣ���Mn02(���н϶��Mn0��MnC03)���ᴿ�ǹ�ҵ��������Ҫ���ڡ���ͼ��ij�о���ѧϰС����ʵ������ģ�ҵ�ϴ�Mn02ת��Ϊ��Mn02�Ĺ������̡�
 ��ش��������⣺
��ش��������⣺
(1)NaCl03����Ԫ�صĻ��ϼ�Ϊ �ۡ�
(2)����A�������� ��
(3)�������������������̨(����Ȧ)�����������ƾ��ƺ� �����в����������� ��
(4)д����MnO2��Ʒ��ϡH2SO4ʱ����C02�Ļ�ѧ����ʽ ��
(5)�������п���ѭ�����õ�������H2SO4�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com